Global Avian Influenza Drug Market
Market Size in USD Million
CAGR :
% 
 USD
774.85 Million
USD
1.25 Million
2024
2032
USD
774.85 Million
USD
1.25 Million
2024
2032
| 2025 –2032 | |
| USD 774.85 Million | |
| USD 1.25 Million | |
|
|
|
|
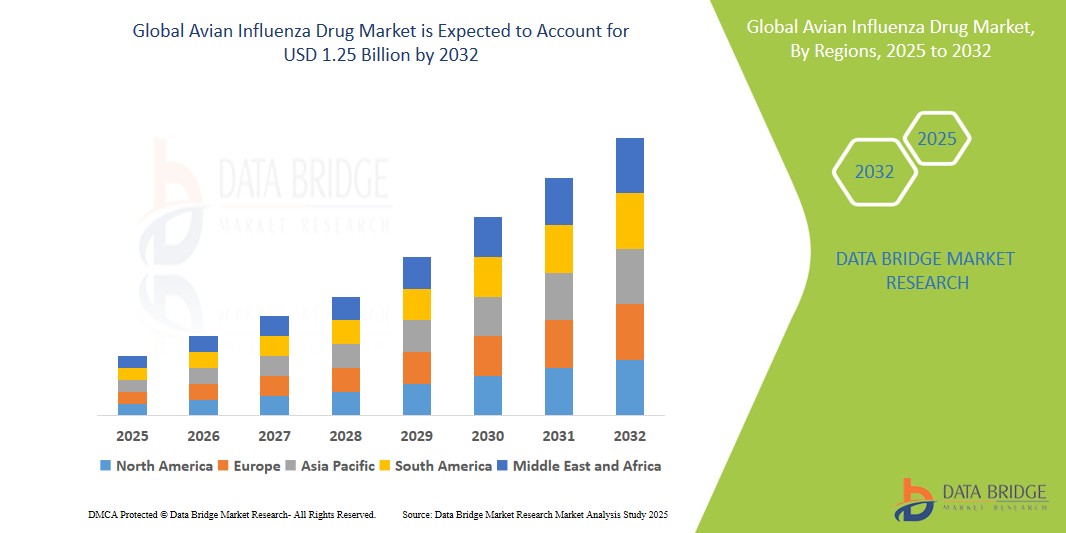
Avian Influenza Drug Market Size
- The global avian influenza vaccines market was valued at USD 774.85 Million in 2024 and is expected to reach USD 1.25 Billion by 2032, registering a CAGR of 7.07% during the forecast period
- This growth is driven by factors such as the rising prevalence of avian influenza outbreaks, increasing awareness about poultry health, and advancements in vaccine technologies
Avian Influenza Drug Market Analysis
- Avian influenza vaccines are crucial in controlling and preventing outbreaks of avian flu, which can significantly affect poultry populations and human health. These vaccines are designed to protect poultry from various strains of the virus, including H5, H7, and H9.
- The demand for these vaccines is primarily driven by the increased incidence of avian influenza outbreaks and the growing need for biosecurity in poultry farming to protect both animal and public health.
- The Asia-Pacific region is expected to dominate the avian influenza vaccines market due to its large poultry farming industry and frequent outbreaks of avian influenza.
- North America and Europe are expected to witness moderate growth, with an increasing focus on vaccination programs to control the spread of avian influenza and prevent economic losses in the poultry industry.
- The H5 strain vaccine segment is expected to hold the largest market share, accounting for 46.9% of the total market, due to the frequent outbreaks of this strain in poultry farms. This leads to increased vaccination demand, as controlling H5 avian influenza is critical for maintaining poultry health and preventing widespread outbreaks.
Report Scope and Avian Influenza Drug Market Segmentation
|
Attributes |
Avian Influenza Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Avian Influenza Drug Market Trends
“Emergence of Novel Vaccine Technologies for Enhanced Protection”
- A significant trend in the avian influenza vaccines market is the emergence of novel vaccine technologies, including recombinant vector vaccines and DNA-based vaccines
- These advanced technologies offer enhanced immunity, longer protection duration, and improved safety profiles, reducing the need for frequent booster doses
- For instance, recent developments in live recombinant vaccines have shown improved efficacy in targeting multiple avian influenza virus strains simultaneously, especially in high-risk poultry regions.
- These innovations are reshaping preventive strategies in poultry farming, improving disease control, and accelerating the adoption of next-generation avian influenza vaccines across global markets.
Avian Influenza Drug Market Dynamics
Driver
“Increasing Frequency of Avian Influenza Outbreaks”
- The growing frequency of avian influenza outbreaks globally is a key factor driving the demand for avian influenza vaccines. Outbreaks have severe consequences for poultry industries, leading to mass culling, trade restrictions, and economic losses.
- Countries with large poultry farming industries are ramping up vaccination programs to prevent viral spread and safeguard food security.
- The global push toward preventive healthcare in animals is also contributing to sustained vaccine demand.
For instance,
- In March 2023, the World Organization for Animal Health (WOAH) reported a surge in H5N1 avian influenza outbreaks across Europe and Asia, prompting mass vaccination efforts to contain the virus.
- As outbreaks become more frequent and widespread, the demand for effective and rapid-response avian influenza vaccines continues to rise, fueling market growth
Opportunity
“Expansion of Government and International Vaccination Programs”
- Global and regional governments, along with organizations like the FAO and WHO, are increasingly supporting avian influenza vaccination as part of biosecurity measures to ensure food supply and prevent zoonotic transmission
- These initiatives provide funding and logistic support for widespread immunization in poultry populations, especially in developing regions with limited access to veterinary services
- The strategic stockpiling of vaccines by governments is also creating opportunities for vaccine manufacturers
For instance,
- In September 2024, the FAO launched a coordinated vaccination campaign across Southeast Asia, targeting high-risk zones with a focus on controlling the spread of H5 and H9 strains through subsidized vaccines and veterinary infrastructure
- Such initiatives not only mitigate the risk of outbreaks but also open new markets for vaccine manufacturers seeking government and institutional contracts.
Restraint/Challenge
“Strain Variability and Vaccine Efficacy Concerns”
- One of the major challenges in the avian influenza vaccines market is the high mutation rate of the virus, leading to strain variability and reduced vaccine efficacy over time
- Constant monitoring and reformulation of vaccines are required to address new and evolving strains, which increases production costs and delays vaccine deployment
- Additionally, incomplete or improperly implemented vaccination campaigns can contribute to viral persistence and mutations
For instance,
- According to a 2022 study published in the journal Veterinary Microbiology, mismatches between circulating H7 strains and available vaccines in certain regions resulted in limited protection, underlining the need for more adaptable vaccine platforms
- These challenges hinder the consistent effectiveness of vaccines, posing a barrier to sustained immunity and potentially limiting market penetration in regions with diverse viral profiles
Avian Influenza Drug Market Scope
The market is segmented on the basis vaccine type, application, and strain.
|
Segmentation |
Sub-Segmentation |
|
By Vaccine Type |
|
|
By Application |
|
|
By Strain |
|
In 2025, the H5 strain vaccine is projected to dominate the market with the largest share in the strain segment
The H5 strain vaccine segment is expected to dominate the global avian influenza vaccines market with the largest share of 47.6% in 2025 due to the high frequency and severity of H5 strain outbreaks in poultry farms worldwide. As one of the most contagious and economically damaging strains, targeted vaccination against H5 is critical for outbreak prevention. Growing government vaccination initiatives and international efforts to curb H5N1 transmission further reinforce the demand, contributing significantly to this segment’s market dominance.
The inactivated vaccines segment is expected to account for the largest share during the forecast period in the vaccine type market
In 2025, the inactivated vaccines segment is projected to lead the avian influenza vaccines market with the largest market share of 53.8%, owing to its proven safety profile and effectiveness in providing strong immune protection. These vaccines are widely used in commercial poultry due to their stability, ease of storage, and minimal risk of reversion to virulence. The increasing implementation of preventive vaccination programs and regulatory approvals for inactivated vaccines across high-risk regions drive the continued growth and dominance of this segment.
Avian Influenza Drug Market Regional Analysis
“Asia-Pacific Holds the Largest Share in the Avian Influenza Vaccines Market”
- Asia-Pacific dominates the global avian influenza vaccines market, primarily due to the high concentration of poultry farming in countries like China, India, Indonesia, and Vietnam, which are frequently affected by avian influenza outbreaks
- China holds a significant share owing to its massive poultry population and proactive government vaccination campaigns aimed at controlling recurring H5 and H9 strain infections
- The region’s dominance is further driven by supportive regulatory frameworks, increasing awareness among poultry farmers, and government-backed mass immunization programs
- High poultry consumption rates, economic dependence on the poultry industry, and rising investment in preventive animal healthcare continue to reinforce the region’s leadership in the market.
“Latin America is Projected to Register the Highest CAGR in the Avian Influenza Vaccines Market”
- Latin America is expected to witness the highest growth rate in the avian influenza vaccines market during the forecast period, fueled by growing poultry production and export activities, especially in countries like Brazil, Argentina, and Mexico
- Brazil, as one of the world’s leading poultry exporters, is increasingly focusing on biosecurity and vaccination strategies to meet international trade requirements and prevent disease outbreaks
- Rising concerns over zoonotic disease transmission and government mandates for avian influenza prevention are encouraging wider vaccine adoption across commercial poultry farms
- Increased investment in animal health infrastructure, growing awareness about disease control, and expanding access to veterinary healthcare in rural and peri-urban regions are expected to further accelerate market growth in this region.
Avian Influenza Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Sanofi (France)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mallinckrodt (US)
- Elanco (US)
- Boehringer Ingelheim International GmbH. (Germany)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Eli Lilly and Company (US)
- Merck & Co., Inc. (US)
- AstraZeneca (UK)
- Johnson & Johnson Private Limited (US)
- Virbac (France)
- Vetoquinol (France)
- Zoetis (US)
- Ceva (France)
- Bimeda, Inc. (US)
- Phibro Animal Health Corporation (US)
- HIPRA (Spain)
- Medion (Germany)
- YEBIO BIOENGINEERING CO., LTD OF QINGDAO (China)
Latest Developments in Global Avian Influenza Drug Market
- In December 2022, Hester Biosciences Ltd., an India-based animal and poultry vaccine manufacturing company, acquired the technology from the Indian Council of Agricultural Research and the National Institute of High-Security Animal Diseases (ICAR-NIHSAD) for an undisclosed amount. With this technology acquisition, the aim of Hester Biosciences acquiring the technology to develop an inactivated H9N2 avian influenza vaccine for poultry is to provide a solution to the significant economic losses faced by Indian poultry farmers due to periodic outbreaks of the disease. The Indian Council of Agricultural Research, National Institute of High Security Animal Diseases (ICAR-NIHSAD), is an India-based research institute that offers technology for aviation flu vaccines and diagnostic tests for avian influenza.
- In February 2024, Zoetis Inc. announced the launch of a new recombinant avian influenza vaccine targeting emerging H5N1 variants. In June 2024, Ceva Santé Animale introduced its Vectormune AI H9 vaccine across Asia-Pacific markets to help manage rising H9N2 outbreaks. Both launches aim to enhance protection against evolving strains and improve poultry health outcomes.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL AVIAN INFLUENZA DRUG MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL AVIAN INFLUENZA DRUG MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL AVIAN INFLUENZA DRUG MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 REGULATORY SCENARIO
6 PREMIUM INSIGHTS
6.1 PESTLE ANALYSIS
6.2 PORTER’S FIVE FORCES
7 INDUSTRY INSIGHTS
8 IMPACT OF COVID-19 PANDEMIC ON THE MARKET
8.1 PRICE IMPACT
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC DECISION FOR MANUFACTURER/SERVICE PROVIDER
8.5 CONCLUSION
9 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY TREATMENT
9.1 OVERVIEW
9.2 DRUGS
9.2.1 OSELTAMIVIR (TAMIFLU)
9.2.2 ZANAMIVIR (RELENZA)
9.2.3 RIMANTADINE
9.2.4 OTHERS
9.3 VACCINES
9.3.1 NON-ADJUVANTED
9.3.2 ADJUVANTED
9.3.2.1. SUBVIRION VACCINES
9.3.2.1.1. ALUMINIUM
9.3.2.1.2. AS03
9.3.2.1.3. MF59
9.3.2.1.4. OTHERS
9.3.2.2. WHOLE-VIRION VACCINES
9.3.2.3. VIROSOMAL VACCINE
9.3.3 MODIFIED VACCINIA VIRUS VECTOR VACCINE
9.3.4 LIVE ATTENUATED VACCINE
9.3.5 OTHERS
9.4 OTHERS
10 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY STRAIN TYPE
10.1 OVERVIEW
10.2 H5N1
10.3 H5N6
10.4 H6N1
10.5 H7N4
10.6 H7N9
10.7 H9N2
10.8 H10N8
10.9 OTHERS
11 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY ROUTE OF ADMINISTRATION
11.1 OVERVIEW
11.2 ORAL
11.3 INJECTABLE
12 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY TYPE
12.1 OVERVIEW
12.2 CHICKEN
12.2.1 HIGHLY PATHOGENIC AVIAN INFLUENZA (HPAI)
12.2.2 LOW PATHOGENIC AVIAN INFLUENZA (LPAI)
12.3 TURKEY
12.4 GOOSE
12.5 DUCK
12.6 OTHERS
13 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS & CLINICS
13.3 HOME CARE SETTING
13.4 ACADEMIC AND RESEARCH INSTITUTES
13.5 OTHERS
14 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
14.4 ONLINE PHARMACY
14.5 OTHERS
15 GLOBAL AVIAN INFLUENZA DRUG MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.2 COMPANY SHARE ANALYSIS: EUROPE
15.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15.4 MERGERS & ACQUISITIONS
15.5 NEW PRODUCT DEVELOPMENT & APPROVALS
15.6 EXPANSIONS
15.7 REGULATORY CHANGES
15.8 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
16 GLOBAL AVIAN INFLUENZA DRUG MARKET, BY REGION
16.1 GLOBAL AVIAN INFLUENZA DRUG MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
16.1.1 NORTH AMERICA
16.1.1.1. U.S.
16.1.1.2. CANADA
16.1.1.3. MEXICO
16.1.2 EUROPE
16.1.2.1. GERMANY
16.1.2.2. FRANCE
16.1.2.3. U.K.
16.1.2.4. ITALY
16.1.2.5. SPAIN
16.1.2.6. RUSSIA
16.1.2.7. TURKEY
16.1.2.8. BELGIUM
16.1.2.9. NETHERLANDS
16.1.2.10. SWITZERLAND
16.1.2.11. REST OF EUROPE
16.1.3 ASIA-PACIFIC
16.1.3.1. JAPAN
16.1.3.2. CHINA
16.1.3.3. SOUTH KOREA
16.1.3.4. INDIA
16.1.3.5. AUSTRALIA
16.1.3.6. SINGAPORE
16.1.3.7. THAILAND
16.1.3.8. MALAYSIA
16.1.3.9. INDONESIA
16.1.3.10. PHILIPPINES
16.1.3.11. REST OF ASIA-PACIFIC
16.1.4 SOUTH AMERICA
16.1.4.1. BRAZIL
16.1.4.2. ARGENTINA
16.1.4.3. REST OF SOUTH AMERICA
16.1.5 MIDDLE EAST AND AFRICA
16.1.5.1. SOUTH AFRICA
16.1.5.2. SAUDI ARABIA
16.1.5.3. UAE
16.1.5.4. EGYPT
16.1.5.5. ISRAEL
16.1.5.6. REST OF MIDDLE EAST AND AFRICA
16.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
17 GLOBAL AVIAN INFLUENZA DRUG MARKET, SWOT AND DBMR ANALYSIS
18 GLOBAL AVIAN INFLUENZA DRUG MARKET, COMPANY PROFILE
18.1 GLAXOSMITHKLINE PLC
18.1.1 COMPANY OVERVIEW
18.1.2 REVENUE ANALYSIS
18.1.3 GEOGRAPHIC PRESENCE
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPEMENTS
18.2 F. HOFFMANN-LA ROCHE LTD
18.2.1 COMPANY OVERVIEW
18.2.2 REVENUE ANALYSIS
18.2.3 GEOGRAPHIC PRESENCE
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPEMENTS
18.3 ALLERGAN
18.3.1 COMPANY OVERVIEW
18.3.2 REVENUE ANALYSIS
18.3.3 GEOGRAPHIC PRESENCE
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPEMENTS
18.4 NOVARTIS AG
18.4.1 COMPANY OVERVIEW
18.4.2 REVENUE ANALYSIS
18.4.3 GEOGRAPHIC PRESENCE
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPEMENTS
18.5 BAXTER
18.5.1 COMPANY OVERVIEW
18.5.2 REVENUE ANALYSIS
18.5.3 GEOGRAPHIC PRESENCE
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPEMENTS
18.6 BIOCRYST PHARMACEUTICALS, INC.
18.6.1 COMPANY OVERVIEW
18.6.2 REVENUE ANALYSIS
18.6.3 GEOGRAPHIC PRESENCE
18.6.4 PRODUCT PORTFOLIO
18.6.5 RECENT DEVELOPEMENTS
18.7 UNM PHARMA INC.
18.7.1 COMPANY OVERVIEW
18.7.2 REVENUE ANALYSIS
18.7.3 GEOGRAPHIC PRESENCE
18.7.4 PRODUCT PORTFOLIO
18.7.5 RECENT DEVELOPEMENTS
18.8 CSL LIMITED
18.8.1 COMPANY OVERVIEW
18.8.2 REVENUE ANALYSIS
18.8.3 GEOGRAPHIC PRESENCE
18.8.4 PRODUCT PORTFOLIO
18.8.5 RECENT DEVELOPEMENTS
18.9 EMERGENT BIOSOLUTIONS INC.
18.9.1 COMPANY OVERVIEW
18.9.2 REVENUE ANALYSIS
18.9.3 GEOGRAPHIC PRESENCE
18.9.4 PRODUCT PORTFOLIO
18.9.5 RECENT DEVELOPEMENTS
18.1 NOVAVAX, INC.
18.10.1 COMPANY OVERVIEW
18.10.2 REVENUE ANALYSIS
18.10.3 GEOGRAPHIC PRESENCE
18.10.4 PRODUCT PORTFOLIO
18.10.5 RECENT DEVELOPEMENTS
18.11 MEDIGEN BIOTECHNOLOGY CORP
18.11.1 COMPANY OVERVIEW
18.11.2 REVENUE ANALYSIS
18.11.3 GEOGRAPHIC PRESENCE
18.11.4 PRODUCT PORTFOLIO
18.11.5 RECENT DEVELOPEMENTS
18.12 BIONDVAX PHARMACEUTICALS LTD.
18.12.1 COMPANY OVERVIEW
18.12.2 REVENUE ANALYSIS
18.12.3 GEOGRAPHIC PRESENCE
18.12.4 PRODUCT PORTFOLIO
18.12.5 RECENT DEVELOPEMENTS
18.13 INOVIO PHARMACEUTICALS
18.13.1 COMPANY OVERVIEW
18.13.2 REVENUE ANALYSIS
18.13.3 GEOGRAPHIC PRESENCE
18.13.4 PRODUCT PORTFOLIO
18.13.5 RECENT DEVELOPEMENTS
18.14 VAXART, INC.
18.14.1 COMPANY OVERVIEW
18.14.2 REVENUE ANALYSIS
18.14.3 GEOGRAPHIC PRESENCE
18.14.4 PRODUCT PORTFOLIO
18.14.5 RECENT DEVELOPEMENTS
18.15 JOHNSON & JOHNSON SERVICES, INC.
18.15.1 COMPANY OVERVIEW
18.15.2 REVENUE ANALYSIS
18.15.3 GEOGRAPHIC PRESENCE
18.15.4 PRODUCT PORTFOLIO
18.15.5 RECENT DEVELOPEMENTS
18.16 SINOVAC
18.16.1 COMPANY OVERVIEW
18.16.2 REVENUE ANALYSIS
18.16.3 GEOGRAPHIC PRESENCE
18.16.4 PRODUCT PORTFOLIO
18.16.5 RECENT DEVELOPEMENTS
18.17 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
18.17.1 COMPANY OVERVIEW
18.17.2 REVENUE ANALYSIS
18.17.3 GEOGRAPHIC PRESENCE
18.17.4 PRODUCT PORTFOLIO
18.17.5 RECENT DEVELOPEMENTS
18.18 CEVA
18.18.1 COMPANY OVERVIEW
18.18.2 REVENUE ANALYSIS
18.18.3 GEOGRAPHIC PRESENCE
18.18.4 PRODUCT PORTFOLIO
18.18.5 RECENT DEVELOPEMENTS
18.19 ZOETIS
18.19.1 COMPANY OVERVIEW
18.19.2 REVENUE ANALYSIS
18.19.3 GEOGRAPHIC PRESENCE
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPEMENTS
18.2 QIANYUANHAO BIOLOGICAL CO., LTD.
18.20.1 COMPANY OVERVIEW
18.20.2 REVENUE ANALYSIS
18.20.3 GEOGRAPHIC PRESENCE
18.20.4 PRODUCT PORTFOLIO
18.20.5 RECENT DEVELOPEMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST RELATED REPORTS
19 RELATED REPORTS
20 QUESTIONNAIRE
21 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.