Global Azacitidine Market
Market Size in USD Billion
CAGR :
% 
 USD
90.24 Billion
USD
165.79 Billion
2024
2032
USD
90.24 Billion
USD
165.79 Billion
2024
2032
| 2025 –2032 | |
| USD 90.24 Billion | |
| USD 165.79 Billion | |
|
|
|
|
Azacitidine Market Size
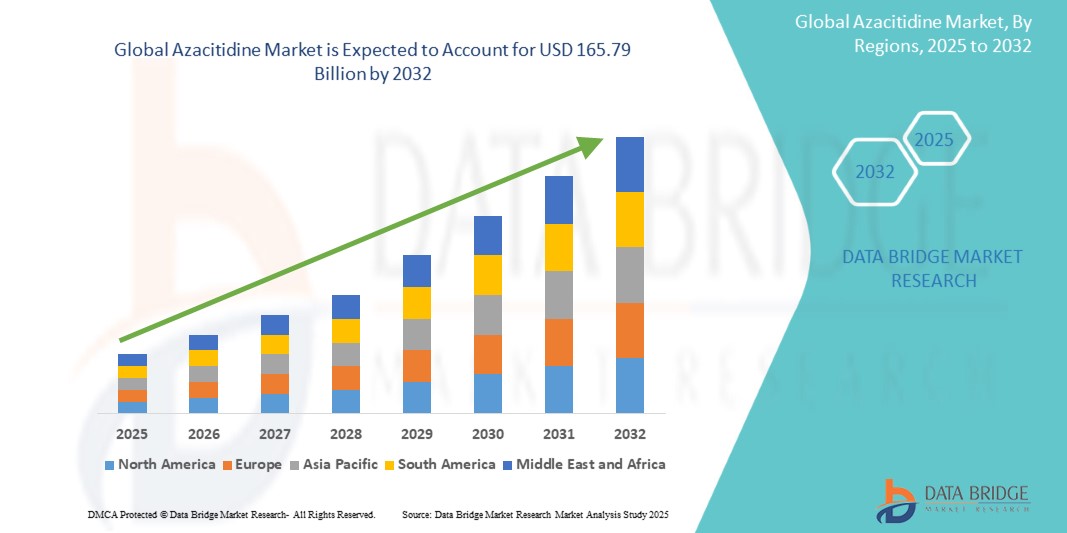
- The global azacitidine market was valued at USD 90.24 billion in 2024 and is expected to reach USD 165.79 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 7.90%, primarily driven by increasing geriatric population
- This growth is driven by factors such as higher disease incidence, longer life expectancy and less intensive treatment need
Azacitidine Market Analysis
- Azacitidine is a hypomethylating agent primarily used in the treatment of myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), and chronic myelomonocytic leukemia (CMML). It plays a crucial role in inhibiting abnormal DNA methylation, thereby restoring normal gene function and slowing disease progression
- The market growth is driven by the rising prevalence of hematological malignancies, increasing adoption of hypomethylating agents (HMAs) in first-line treatment, and the expanding geriatric population, which is more susceptible to these disorders
- In addition, innovations in drug delivery methods, such as the development of oral azacitidine (Onureg), are transforming treatment approaches by enhancing patient compliance and reducing hospital visits
- For instance, the approval of Onureg (oral azacitidine) has provided an alternative to injectable formulations, allowing for greater flexibility in treatment administration
Report Scope and Azacitidine Market Segmentation
|
Attributes |
Azacitidine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Azacitidine Market Trends
“Growing Adoption of Oral Azacitidine Formulations”
- One prominent trend in the global azacitidine market is the growing adoption of oral azacitidine formulations
- This shift is driven by the demand for improved patient convenience, reduced hospital visits, and enhanced treatment adherence, encouraging pharmaceutical companies to develop advanced oral formulations for better disease management
- For instance, Bristol Myers Squibb introduced Onureg (oral azacitidine), which offers a more convenient administration route while maintaining therapeutic efficacy for patients with acute myeloid leukemia (AML) in remission
- As healthcare systems prioritize patient-friendly treatment options, the azacitidine market is evolving with innovations in drug formulation, extended-release mechanisms, and combination therapies, ensuring better treatment outcomes and wider accessibility
- This shift is expected to drive market expansion as oral hypomethylating agents (HMAs) gain traction in oncology treatment protocols
Azacitidine Market Dynamics
Driver
“Rising Prevalence of Hematological Disorders”
- The increasing prevalence of hematological disorders, particularly myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), and chronic myelomonocytic leukemia (CMML), is a key driver of growth in the azacitidine market
- As the incidence of these disorders rises, the demand for effective hypomethylating agents (HMAs) such as azacitidine continues to grow, making it a crucial treatment option for managing these conditions
- This shift is particularly evident in North America, Europe, and Asia-Pacific, where aging populations, improved diagnostic capabilities, and greater access to advanced therapies are fueling market expansion. With a growing number of patients requiring long-term disease management, azacitidine is increasingly preferred for its ability to delay disease progression and improve survival outcomes
- With a growing number of patients requiring long-term disease management, azacitidine is increasingly preferred for its ability to delay disease progression and improve survival outcomes
- To meet this rising demand, pharmaceutical companies are investing in innovative formulations, combination therapies, and expanded treatment indications, ensuring broader access to effective hematological care
For instance,
- Takeda Pharmaceutical Company has been exploring combination therapies involving azacitidine and venetoclax for enhanced treatment efficacy in AML patients who cannot tolerate intensive chemotherapy
- Ongoing clinical research by Eisai Co., Ltd. and Daiichi Sankyo is evaluating azacitidine in combination with targeted agents to improve response rates in MDS and AML
- With increasing healthcare investments and ongoing research into next-generation HMAs, targeted therapies, and personalized treatment approaches, the azacitidine market is poised for sustained growth, offering improved therapeutic options and better survival rates for patients with hematological malignancies worldwide
Opportunity
“Rising Awareness About Myelodysplastic Syndrome (MDS)”
- The growing awareness of myelodysplastic syndrome (MDS) presents a significant opportunity for the Azacitidine market, as increased disease recognition leads to earlier diagnosis, higher treatment rates, and improved patient outcomes.
- Public health initiatives, medical conferences, and awareness campaigns are educating both healthcare professionals and patients, driving demand for effective treatment options such as azacitidine
- Pharmaceutical companies and healthcare organizations are investing in educational programs, patient advocacy groups, and research collaborations to enhance MDS awareness and encourage timely diagnosis and treatment
For instance,
- MDS Foundation and Leukemia & Lymphoma Society (LLS) have launched global initiatives to educate patients and clinicians on early symptoms, diagnostic approaches, and available treatment options, increasing azacitidine adoption
- Companies such as Bristol Myers Squibb and Takeda Pharmaceutical are actively engaging in awareness campaigns and collaborating with healthcare providers to expand access to azacitidine therapy
- As MDS awareness continues to rise, the demand for effective treatment solutions such as azacitidine will grow, creating new opportunities for market expansion. With increasing patient education, advancements in diagnostic technologies, and strong industry collaborations, the azacitidine market is well-positioned to benefit from this evolving landscape
Restraint/Challenge
“Late Approvals from Regulatory Organizations”
- Delays in regulatory approvals pose a significant challenge for the azacitidine market, as prolonged evaluation timelines can hinder new drug formulations, expanded indications, and combination therapies from reaching patients in a timely manner
- Regulatory agencies such as the U.S. FDA, EMA, and other national health authorities require extensive clinical data, leading to long approval cycles that slow market expansion
- Strict regulatory requirements and the need for comprehensive safety and efficacy trials often result in delayed drug launches and limited early access to new therapies. This challenge is particularly evident in regions with stringent approval processes, where pharmaceutical companies must navigate complex compliance frameworks before introducing updated formulations or label extensions
For instance,
- The approval process for oral azacitidine (Onureg) faced delays due to the need for additional clinical trial data on long-term efficacy and safety, slowing its market penetration in certain countries
- As regulatory requirements become more rigorous, pharmaceutical manufacturers must invest in extensive clinical trials, real-world evidence studies, and regulatory compliance strategies to accelerate approval timelines and ensure faster patient access to innovative azacitidine-based therapies
Azacitidine Market Scope
The market is segmented on the basis of type, product, application, route of administration, distribution channel, and end-user.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Product |
|
|
By Application |
|
|
By Route of Administration |
|
|
By Distribution Channel |
|
|
By End-User |
|
Azacitidine Market Regional Analysis
“North America is the Dominant Region in the Azacitidine Market”
- North America dominates the azacitidine market, driven by the high healthcare expenditures, a well-established healthcare infrastructure, and strong research and development investments. The region continues to lead in drug innovation, regulatory approvals, and early adoption of novel treatment approaches for hematological disorders
- The U.S. holds a significant share due to rising prevalence of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), increasing geriatric population, and high adoption of hypomethylating agents (HMAs) such as azacitidine
- Leading pharmaceutical companies such as Bristol Myers Squibb, Pfizer, and Takeda Pharmaceutical are investing heavily in new drug formulations, clinical trials, and combination therapies to expand the therapeutic applications of azacitidine
- In addition, ongoing advancements in personalized medicine, biomarker-based treatment strategies, and AI-assisted drug development are accelerating North America’s leadership in the azacitidine market, ensuring improved treatment efficacy and patient outcomes
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is expected to witness the highest growth rate in the azacitidine market, driven by increasing consumption demand for active pharmaceutical ingredients (APIs), rising healthcare expenditures, and improving access to cancer treatments.
- Countries such as China, India, Japan, and South Korea are witnessing a surge in demand for hematological cancer therapies, supported by growing awareness of myelodysplastic syndromes (MDS), improving diagnostic capabilities, and government initiatives to enhance cancer care
- Global pharmaceutical companies are expanding their presence in the region through strategic partnerships with local drug manufacturers and healthcare institutions, enabling faster drug approvals and market penetration
- Furthermore, advancements in biopharmaceutical research, increased adoption of oral azacitidine formulations, and integration of AI-driven drug discovery are transforming the treatment landscape, positioning Asia-Pacific as the fastest-growing market for azacitidine-based therapies over the forecast period
Azacitidine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Novartis AG (Switzerland)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Private Limited (U.S.)
- Abbott (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bausch Health Companies Inc. (Canada)
- AstraZeneca (U.K.)
- GSK plc (U.K.)
- H. Lundbeck A/S (Denmark)
- Takeda Pharmaceutical Company Limited (Japan)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Eisai Co., Ltd (Japan)
- Merz Pharma (Germany)
Latest Developments in Global Azacitidine Market
-
In September 2024, Intas Pharmaceuticals Ltd. announced the launch of Azadine-O, the first-ever oral Azacitidine therapy for Acute Myeloid Leukemia (AML) in the Indian market. This groundbreaking development marks a major milestone in the Azacitidine market, providing patients with a more convenient and patient-friendly alternative to traditional injectable formulations
- In May 2022, the U.S. Food and Drug Administration (FDA) granted approval for azacitidine (Vidaza, Celgene Corp.) for use in pediatric patients with newly diagnosed juvenile myelomonocytic leukemia (JMML). This approval marked a significant milestone in the Azacitidine market, expanding its therapeutic application beyond myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) to address a rare and aggressive form of childhood leukemia
- In September 2020, Hikma Pharmaceuticals PLC, a leading multinational pharmaceutical company, announced the launch of Azacitidine for Injection, 100mg, the generic version of Vidaza®, in the United States through its US affiliate, Hikma Pharmaceuticals USA Inc. This launch marked a significant development in the Azacitidine market, enhancing accessibility and affordability of the drug for patients suffering from myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). The introduction of a cost-effective generic alternative increased market competition, driving greater adoption of Azacitidine-based therapies across healthcare providers
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL AZACITIDINE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL AZACITIDINE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL AZACITIDINE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR AZACITIDINE MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR AZACITIDINE MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR AZACITIDINE MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR AZACITIDINE MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR AZACITIDINE MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
18 GLOBAL AZACITIDINE MARKET, BY TYPES
18.1 OVERVIEW
18.2 0.995
18.3 <99.5%
18.4 OTHERS
19 GLOBAL AZACITIDINE MARKET, BY DOSAGE
19.1 OVERVIEW
19.2 STANDARD SYRINGES
19.3 PRE-FILLED SYRINGES
20 GLOBAL AZACITIDINE MARKET, BY STRENGTH
20.1 OVERVIEW
20.2 TABLET
20.2.1 200MG
20.2.2 300MG
20.2.3 OTHERS
20.3 INJECTION
20.3.1 25 MG/ML POWDER FOR INJECTION
20.3.2 100MG/SINGLE-DOSE VIAL INJECTION
20.3.3 OTHERS
21 GLOBAL AZACITIDINE MARKET, BY ROUTE OF ADMINISTRATION
21.1 OVERVIEW
21.2 ORAL
21.2.1 TABLETS
21.2.2 OTHERS
21.3 PARENTERAL
21.3.1 INTRAVENEOUS
21.3.2 SUBCUTANEOUS
21.3.3 OTHERS
21.4 OTHERS
22 GLOBAL AZACITIDINE MARKET, BY DRUG TYPE
22.1 OVERVIEW
22.2 BRANDED
22.2.1 VIDAZA
22.2.2 ONUREG
22.2.3 OTHERS
22.3 GENERICS
23 GLOBAL AZACITIDINE MARKET, BY AGE GROUP
23.1 OVERVIEW
23.2 PEDIATRIC
23.3 ADULT
23.4 GERIATRIC
24 GLOBAL AZACITIDINE MARKET, BY GEDNER
24.1 OVERVIEW
24.2 MALE
24.2.1 PEDIATRIC
24.2.2 ADULT
24.2.3 GERIATRIC
24.3 FEMALE
24.3.1 PEDIATRIC
24.3.2 ADULT
24.3.3 GERIATRIC
25 GLOBAL AZACITIDINE MARKET, BY APPLICATION
25.1 OVERVIEW
25.2 REFRACTORY ANEMIA (RA)
25.3 REFRACTORY ANEMIA WITH EXCESS BLASTS (RAEB)
25.4 MYELODYSPLASTIC SYNDROMES
25.5 CHRONIC MYELOMONOCYTIC LEUKEMIA (CMMOL)
25.6 ACUTE MYELOID LEUKEMIA
25.7 OTHERS
26 GLOBAL AZACITIDINE MARKET, BY END USER
26.1 OVERVIEW
26.2 HOSPITALS
26.2.1 BY TYPE
26.2.1.1. PUBLIC
26.2.1.2. PRIVATE
26.2.2 BY LEVEL
26.2.2.1. TIER 1
26.2.2.2. TIER 2
26.2.2.3. TIER 3
26.3 SPECIALTY CLINICS
26.3.1 PUBLIC
26.3.2 PRIVATE
26.4 ONCOLOGY CENTRES
26.5 HOME HEALTHCARE
26.6 ACADEMIC AND RESEARCH INSTITUTION
26.7 OTHERS
27 GLOBAL AZACITIDINE MARKET, BY DISTRIBUTION CHANNEL
27.1 OVERVIEW
27.2 DIRECT TENDER
27.3 RETAIL SALES
27.3.1 ONLINE
27.3.1.1. COMPANY WEBSITE
27.3.1.2. E-DRUG STORES
27.3.1.3. OTHERS
27.3.2 OFFLINE
27.3.2.1. HOSPITAL PHARMACY
27.3.2.2. MEDICINE STORES
27.3.2.3. OTHERS
27.4 OTHERS
28 GLOBAL AZACITIDINE MARKET, COMPANY LANDSCAPE
28.1 COMPANY SHARE ANALYSIS: GLOBAL
28.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
28.3 COMPANY SHARE ANALYSIS: EUROPE
28.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
28.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
28.6 COMPANY SHARE ANALYSIS: SOUTH AMERICA
28.7 MERGERS & ACQUISITIONS
28.8 NEW PRODUCT DEVELOPMENT & APPROVALS
28.9 EXPANSIONS
28.1 REGULATORY CHANGES
28.11 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
29 GLOBAL AZACITIDINE MARKET, BY REGION
29.1 GLOBAL AZACITIDINE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
29.1.1 NORTH AMERICA
29.1.1.1. U.S.
29.1.1.2. CANADA
29.1.1.3. MEXICO
29.1.2 EUROPE
29.1.2.1. GERMANY
29.1.2.2. FRANCE
29.1.2.3. U.K.
29.1.2.4. ITALY
29.1.2.5. SPAIN
29.1.2.6. RUSSIA
29.1.2.7. TURKEY
29.1.2.8. BELGIUM
29.1.2.9. NETHERLANDS
29.1.2.10. HUNGARY
29.1.2.11. LITHUANIA
29.1.2.12. AUSTRIA
29.1.2.13. IRELAND
29.1.2.14. NORWAY
29.1.2.15. POLAND
29.1.2.16. SWITZERLAND
29.1.2.17. REST OF EUROPE
29.1.3 ASIA-PACIFIC
29.1.3.1. JAPAN
29.1.3.2. CHINA
29.1.3.3. SOUTH KOREA
29.1.3.4. INDIA
29.1.3.5. AUSTRALIA
29.1.3.6. SINGAPORE
29.1.3.7. THAILAND
29.1.3.8. MALAYSIA
29.1.3.9. INDONESIA
29.1.3.10. VIETNAM
29.1.3.11. PHILIPPINES
29.1.3.12. REST OF ASIA-PACIFIC
29.1.4 SOUTH AMERICA
29.1.4.1. BRAZIL
29.1.4.2. ARGENTINA
29.1.4.3. PERU
29.1.4.4. REST OF SOUTH AMERICA
29.1.5 MIDDLE EAST AND AFRICA
29.1.5.1. SOUTH AFRICA
29.1.5.2. SAUDI ARABIA
29.1.5.3. UAE
29.1.5.4. EGYPT
29.1.5.5. KUWAIT
29.1.5.6. ISRAEL
29.1.5.7. REST OF MIDDLE EAST AND AFRICA
29.1.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
30 GLOBAL AZACITIDINE MARKET, SWOT AND DBMR ANALYSIS
31 GLOBAL AZACITIDINE MARKET, COMPANY PROFILE
31.1 VBSHILPA
31.1.1 COMPANY OVERVIEW
31.1.2 REVENUE ANALYSIS
31.1.3 GEOGRAPHIC PRESENCE
31.1.4 PRODUCT PORTFOLIO
31.1.5 RECENT DEVELOPEMENTS
31.2 BRISTOL-MYERS SQUIBB COMPANY
31.2.1 COMPANY OVERVIEW
31.2.2 REVENUE ANALYSIS
31.2.3 GEOGRAPHIC PRESENCE
31.2.4 PRODUCT PORTFOLIO
31.2.5 RECENT DEVELOPEMENTS
31.3 HIKMA PHARMACEUTICALS PLC
31.3.1 COMPANY OVERVIEW
31.3.2 REVENUE ANALYSIS
31.3.3 GEOGRAPHIC PRESENCE
31.3.4 PRODUCT PORTFOLIO
31.3.5 RECENT DEVELOPEMENTS
31.4 INTAS PHARMACEUTICALS LIMITED
31.4.1 COMPANY OVERVIEW
31.4.2 REVENUE ANALYSIS
31.4.3 GEOGRAPHIC PRESENCE
31.4.4 PRODUCT PORTFOLIO
31.4.5 RECENT DEVELOPEMENTS
31.5 ACCORD HEALTHCARE
31.5.1 COMPANY OVERVIEW
31.5.2 REVENUE ANALYSIS
31.5.3 GEOGRAPHIC PRESENCE
31.5.4 PRODUCT PORTFOLIO
31.5.5 RECENT DEVELOPEMENTS
31.6 ACTAVIS LLC
31.6.1 COMPANY OVERVIEW
31.6.2 REVENUE ANALYSIS
31.6.3 GEOGRAPHIC PRESENCE
31.6.4 PRODUCT PORTFOLIO
31.6.5 RECENT DEVELOPEMENTS
31.7 AMNEAL PHARMACEUTICALS LLC.
31.7.1 COMPANY OVERVIEW
31.7.2 REVENUE ANALYSIS
31.7.3 GEOGRAPHIC PRESENCE
31.7.4 PRODUCT PORTFOLIO
31.7.5 RECENT DEVELOPEMENTS
31.8 CIPLA
31.8.1 COMPANY OVERVIEW
31.8.2 REVENUE ANALYSIS
31.8.3 GEOGRAPHIC PRESENCE
31.8.4 PRODUCT PORTFOLIO
31.8.5 RECENT DEVELOPEMENTS
31.9 DR. REDDY’S LABORATORIES LIMITED
31.9.1 COMPANY OVERVIEW
31.9.2 REVENUE ANALYSIS
31.9.3 GEOGRAPHIC PRESENCE
31.9.4 PRODUCT PORTFOLIO
31.9.5 RECENT DEVELOPEMENTS
31.1 EUGIA US LLC
31.10.1 COMPANY OVERVIEW
31.10.2 REVENUE ANALYSIS
31.10.3 GEOGRAPHIC PRESENCE
31.10.4 PRODUCT PORTFOLIO
31.10.5 RECENT DEVELOPEMENTS
31.11 EUROHLTH INTL SARL
31.11.1 COMPANY OVERVIEW
31.11.2 REVENUE ANALYSIS
31.11.3 GEOGRAPHIC PRESENCE
31.11.4 PRODUCT PORTFOLIO
31.11.5 RECENT DEVELOPEMENTS
31.12 JIANGSU HANSOH PHARM
31.12.1 COMPANY OVERVIEW
31.12.2 REVENUE ANALYSIS
31.12.3 GEOGRAPHIC PRESENCE
31.12.4 PRODUCT PORTFOLIO
31.12.5 RECENT DEVELOPEMENTS
31.13 MEITHEAL
31.13.1 COMPANY OVERVIEW
31.13.2 REVENUE ANALYSIS
31.13.3 GEOGRAPHIC PRESENCE
31.13.4 PRODUCT PORTFOLIO
31.13.5 RECENT DEVELOPEMENTS
31.14 MSN LABS PVT LTD
31.14.1 COMPANY OVERVIEW
31.14.2 REVENUE ANALYSIS
31.14.3 GEOGRAPHIC PRESENCE
31.14.4 PRODUCT PORTFOLIO
31.14.5 RECENT DEVELOPEMENTS
31.15 NATCO PHARMA LTD
31.15.1 COMPANY OVERVIEW
31.15.2 REVENUE ANALYSIS
31.15.3 GEOGRAPHIC PRESENCE
31.15.4 PRODUCT PORTFOLIO
31.15.5 RECENT DEVELOPEMENTS
31.16 FLORENCIA HEALTHCARE
31.16.1 COMPANY OVERVIEW
31.16.2 REVENUE ANALYSIS
31.16.3 GEOGRAPHIC PRESENCE
31.16.4 PRODUCT PORTFOLIO
31.16.5 RECENT DEVELOPEMENTS
31.17 GETWELL PHARMA
31.17.1 COMPANY OVERVIEW
31.17.2 REVENUE ANALYSIS
31.17.3 GEOGRAPHIC PRESENCE
31.17.4 PRODUCT PORTFOLIO
31.17.5 RECENT DEVELOPEMENTS
31.18 TAJ PHARMA GROUP
31.18.1 COMPANY OVERVIEW
31.18.2 REVENUE ANALYSIS
31.18.3 GEOGRAPHIC PRESENCE
31.18.4 PRODUCT PORTFOLIO
31.18.5 RECENT DEVELOPEMENTS
31.19 HETERO HEALTHCARE LIMITED
31.19.1 COMPANY OVERVIEW
31.19.2 REVENUE ANALYSIS
31.19.3 GEOGRAPHIC PRESENCE
31.19.4 PRODUCT PORTFOLIO
31.19.5 RECENT DEVELOPEMENTS
31.2 ABBOTT
31.20.1 COMPANY OVERVIEW
31.20.2 REVENUE ANALYSIS
31.20.3 GEOGRAPHIC PRESENCE
31.20.4 PRODUCT PORTFOLIO
31.20.5 RECENT DEVELOPEMENTS
31.21 APIS LABOR GMBH (MARKETING AUTH.- MYLAN IRELAND LIMITED )
31.21.1 COMPANY OVERVIEW
31.21.2 REVENUE ANALYSIS
31.21.3 GEOGRAPHIC PRESENCE
31.21.4 PRODUCT PORTFOLIO
31.21.5 RECENT DEVELOPEMENTS
31.22 BRECKENRIDGE PHARMACEUTICAL, INC.
31.22.1 COMPANY OVERVIEW
31.22.2 REVENUE ANALYSIS
31.22.3 GEOGRAPHIC PRESENCE
31.22.4 PRODUCT PORTFOLIO
31.22.5 RECENT DEVELOPEMENTS
31.23 ARMAS PHARMACEUTICALS, INC.
31.23.1 COMPANY OVERVIEW
31.23.2 REVENUE ANALYSIS
31.23.3 GEOGRAPHIC PRESENCE
31.23.4 PRODUCT PORTFOLIO
31.23.5 RECENT DEVELOPEMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
32 RELATED REPORTS
33 CONCLUSION
34 QUESTIONNAIRE
35 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.