Global Balamuthia Infection Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
187.02 Million
USD
253.98 Million
2025
2033
USD
187.02 Million
USD
253.98 Million
2025
2033
| 2026 –2033 | |
| USD 187.02 Million | |
| USD 253.98 Million | |
|
|
|
|
Balamuthia Infection Treatment Market Size
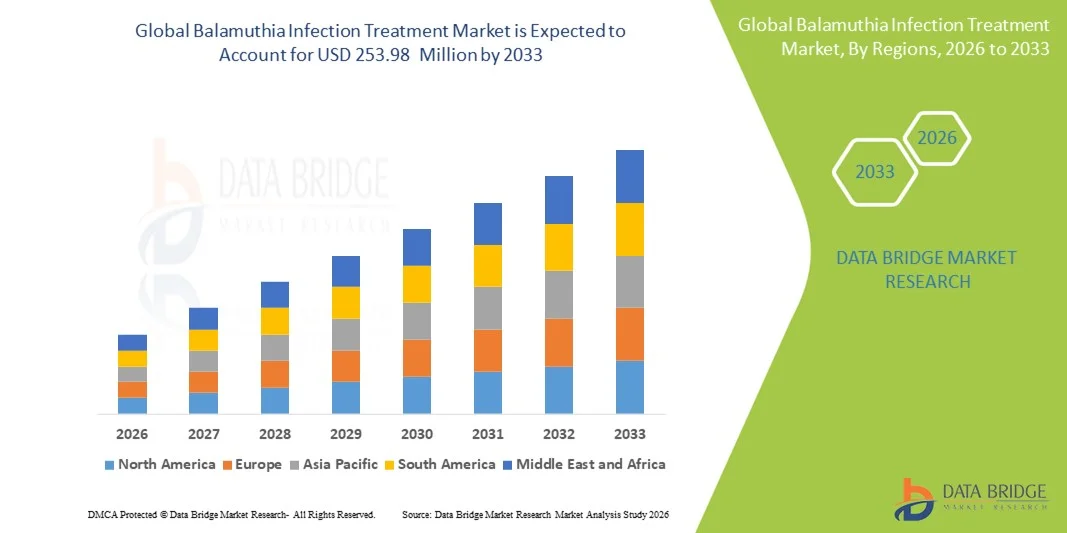
- The global Balamuthia infection treatment market size was valued at USD 187.02 million in 2025 and is expected to reach USD 253.98 million by 2033, at a CAGR of 3.90% during the forecast period
- The market growth is largely driven by increased awareness, improving diagnostic capabilities, and the rising incidence of Balamuthia mandrillaris infections, which have historically been underdiagnosed due to limited clinical recognition and testing accessibility
- Furthermore, expanding research initiatives, enhanced antimicrobial therapy developments, and greater prioritization of rare infectious disease management are strengthening treatment pipelines, thereby accelerating the adoption of specialized therapeutics and boosting overall market expansion
Balamuthia Infection Treatment Market Analysis
- Balamuthia infection treatment, centered on managing the rare but severe Balamuthia mandrillaris infections affecting the central nervous system and skin, is becoming increasingly critical due to rising diagnostic awareness, improved clinical recognition, and the urgent need for effective therapeutic protocols to reduce historically high mortality rates
- The growing demand for Balamuthia infection treatment is primarily driven by increasing global surveillance of rare amoebic infections, improved access to laboratory testing, and heightened clinician awareness, along with expanding research efforts focused on combination antimicrobial regimens and earlier intervention strategies
- North America dominated the Balamuthia infection treatment market with the largest revenue share of 42.3% in 2025, supported by advanced healthcare infrastructure, strong laboratory capabilities, and higher reported case identification, with the U.S. experiencing the most substantial diagnostic improvements due to enhanced infectious disease reporting systems and availability of investigational treatment pathways
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, driven by rising population exposure in tropical climates, improving hospital diagnostic capabilities, and strengthened public health initiatives that are helping to identify and treat rare parasitic infections more effectively
- The Antiprotozoal treatment segment dominated the global Balamuthia infection treatment market with a market share of 38.9% in 2025, supported by the widespread clinical use of multi-drug antiprotozoal regimens including miltefosine and related therapies
Report Scope and Balamuthia Infection Treatment Market Segmentation
|
Attributes |
Balamuthia Infection Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Balamuthia Infection Treatment Market Trends
Growing Adoption of Advanced Diagnostics and Combination Therapy Approaches
- A significant and accelerating trend in the global Balamuthia infection treatment market is the expanding adoption of advanced diagnostic tools such as PCR and immunofluorescence assays, which are improving early detection and enabling faster therapeutic intervention for this rare but highly fatal infection
- For instance, PCR-based diagnostic panels introduced by several reference laboratories now allow clinicians to identify Balamuthia mandrillaris within hours, providing a substantial improvement over older biopsy-dependent methods that often caused treatment delays
- AI-supported diagnostic platforms are also emerging, enabling digital pathology systems to enhance amoebic identification accuracy and supporting clinicians in distinguishing Balamuthia infections from similar neurological or dermatological conditions; for instance, advanced imaging algorithms can flag abnormal tissue patterns suggesting early infection
- The growing reliance on multi-drug antimicrobial regimens is reshaping treatment strategies, allowing clinicians to combine antiprotozoal, antifungal, and antibacterial agents based on disease severity, patient response, and updated clinical guidance for aggressive early-stage management
- This trend toward more precise, technology-driven diagnostics and coordinated therapeutic protocols is fundamentally reshaping expectations for rare infection care, prompting pharmaceutical and diagnostic providers to expand research in targeted drug combinations and rapid detection platforms
- The demand for treatments that integrate rapid diagnostics, broader drug accessibility, and improved clinical decision support is increasing across both developed and emerging regions, as healthcare systems prioritize earlier detection and intervention to improve survival outcomes
Balamuthia Infection Treatment Market Dynamics
Driver
Increasing Clinical Awareness and Rising Adoption of Advanced Diagnostic Testing
- The increasing recognition of Balamuthia mandrillaris infections among clinicians, combined with the rapid adoption of advanced diagnostic technologies across hospitals and laboratories, is a significant driver accelerating the global demand for effective treatment strategies
- For instance, in 2025, several national health laboratories announced expansions of their amoebic infection panels to include Balamuthia-specific PCR and IFA testing, enabling faster case identification and promoting early clinical intervention in suspected encephalitis cases
- As medical professionals become more aware of the high mortality associated with delayed diagnosis, the availability of rapid testing, improved imaging, and broad-spectrum antimicrobial regimens is elevating treatment uptake and strengthening clinical confidence in managing this rare infection
- Furthermore, the rising concern over emergent parasitic and rare infections is increasing the need for specialized therapeutic options, with hospitals integrating Balamuthia testing into neuroinfectious disease protocols to detect cases earlier and improve clinical outcomes
- The inclusion of key drugs such as miltefosine in hospital formularies, coupled with expanded access through global health partnerships, is propelling treatment adoption across both high-risk and general patient populations, particularly in regions with higher environmental exposure
- The growing support for clinician education, improved diagnostic accessibility, and enhanced treatment pathways is steadily driving the expansion of the Balamuthia infection treatment market worldwide
Restraint/Challenge
Limited Drug Availability and Regulatory Barriers for Rare Infection Therapies
- The extremely limited availability of approved therapeutic options, coupled with the dependence on off-label or investigational drug combinations, presents a significant challenge to widespread treatment adoption for Balamuthia infections across global healthcare systems
- For instance, miltefosine one of the cornerstone drugs used in treatment is still restricted in several countries due to regulatory hurdles, requiring special access programs that can delay patient treatment during critical early infection stages
- Concerns about inconsistent access to advanced diagnostic tools such as PCR and IFA, particularly in low-resource regions, further limit timely diagnosis, causing delayed treatment initiation and reducing survival such likely among infected patients
- Addressing these obstacles requires greater regulatory support for investigational antimicrobials, improved global distribution networks, and expanded funding to strengthen diagnostic capacity across hospitals and specialized laboratories
- In addition, the absence of standardized global treatment guidelines and the reliance on case-by-case therapeutic decisions contribute to clinical uncertainty, especially for healthcare facilities with limited experience managing rare amoebic infections
- Overcoming these challenges through increased research investment, streamlined global regulatory pathways, and broader clinician training will be essential for improving treatment accessibility and supporting long-term market growth
Balamuthia Infection Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, dosage, route of administration, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the Balamuthia infection treatment market is segmented into antifungal, antiprotozoal, anthelmintic, and others. The Antiprotozoal segment dominated the market with the largest market revenue share of 38.9% in 2025, driven by its central role in multi-drug treatment regimens for Balamuthia mandrillaris infections. Miltefosine and other antiparasitic agents are preferred due to oral availability, case-report evidence, and inclusion in hospital formularies. Hospitals often prioritize antiprotozoals for their established efficacy and availability in compassionate-use programs. The market also sees strong demand for antiprotozoal treatments due to their use in combination therapies with antifungals and antibiotics. Clinical familiarity and global distribution channels further reinforce the dominance of this segment.
The Antifungal segment is anticipated to witness the fastest growth rate of 24.1% from 2026 to 2033, fueled by increasing adoption in combination regimens and growing clinical evidence supporting survival benefits. Antifungals such as azoles and investigational nitroxoline are being integrated into treatment protocols alongside antiprotozoals. Improved formulation availability and hospital adoption for severe cases also support market growth. The rising number of case reports highlighting antifungal efficacy drives awareness among infectious disease specialists. Hospitals and reference laboratories are scaling antifungal use in multi-drug therapies. The increasing R&D focus on antifungals for rare CNS infections further accelerates adoption.
- By Diagnosis
On the basis of diagnosis, the Balamuthia infection treatment market is segmented into indirect immunofluorescence assay (IFA), biopsy, polymerase chain reaction (PCR), and others. The Biopsy segment dominated the market with the largest market revenue share of 45.2% in 2025, driven by its established role as the definitive diagnostic method. Histopathological analysis allows direct visualization of trophozoites and granulomatous lesions. Hospitals often prioritize biopsy for accurate diagnosis and treatment planning. The market also sees strong demand due to its reliability in differentiating Balamuthia from other CNS infections. Pathology laboratories and surgical teams contribute significantly to the biopsy market share. Clinical guidelines continue to emphasize biopsy as the gold standard for diagnosis.
The PCR segment is anticipated to witness the fastest growth rate of 29.3% from 2026 to 2033, fueled by its rapid and sensitive detection capabilities. PCR enables early identification of Balamuthia DNA in CSF or tissue samples. Laboratories and reference centers increasingly adopt PCR panels to improve diagnostic speed. PCR reduces reliance on invasive biopsies and shortens time-to-treatment initiation. Growing adoption in tertiary care hospitals and improved access to molecular diagnostics contribute to market growth. The ease of integration into multiplex encephalitis panels further supports the rapid uptake of PCR-based diagnostics.
- By Dosage
On the basis of dosage, the Balamuthia infection treatment market is segmented into cream, tablet, injection, and others. The Tablet segment dominated the market with the largest market revenue share of 52.6% in 2025, driven by oral administration of miltefosine and other antiparasitic agents. Tablets are preferred for outpatient continuation after initial inpatient stabilization. Hospitals often prioritize tablets for long-term multi-drug therapy regimens. The market also sees strong demand due to ease of distribution and patient adherence. Clinical familiarity with oral dosing schedules reinforces tablet dominance. Tablets remain central to most published treatment protocols.
The Injection segment is anticipated to witness the fastest growth rate of 26.8% from 2026 to 2033, fueled by increased adoption in severe CNS cases. Intravenous administration allows higher plasma and CNS drug concentrations. Hospitals adopt IV therapy for rapid dose escalation in critical patients. ICU protocols often rely on IV delivery to maximize therapeutic effect. Hospital pharmacy and parenteral compounding capacity support the rising use of injections. New case reports and guidelines advocating early IV therapy further drive growth.
- By Route of Administration
On the basis of route of administration, the Balamuthia infection treatment market is segmented into oral, topical, intravenous, and others. The Oral segment dominated the market with the largest market revenue share of 54.0% in 2025, driven by oral dosing of miltefosine in most treatment regimens. Oral therapy supports long-term outpatient management. Hospitals often rely on oral therapy after stabilization. The market also sees strong demand due to convenience and adherence benefits. Oral dosing is central to combination therapy strategies. Clinical practice patterns reinforce oral as the largest route of administration.
The Intravenous segment is anticipated to witness the fastest growth rate of 27.1% from 2026 to 2033, fueled by rapid administration requirements in critical CNS infections. IV therapy allows higher drug levels in plasma and CNS. Hospitals adopt IV routes for early aggressive therapy. ICU protocols and parenteral compounding services support IV adoption. Case reports demonstrate improved outcomes with IV adjunctive therapy. The growth rate of IV administration surpasses oral despite oral dominance in absolute volumes.
- By End-Users
On the basis of end-users, the Balamuthia infection treatment market is segmented into clinic, hospital, and others. The Hospital segment dominated the market with the largest market revenue share of 61.3% in 2025, driven by inpatient care requirements, neurosurgical procedures, and intensive multi-drug management. Hospitals often prioritize treatment for severe cases requiring diagnostic confirmation and acute therapy. The market also sees strong demand due to hospital pharmacy capabilities and specialist teams. Referral patterns concentrate suspected cases in hospitals. Hospital dominance is reinforced by length of stay and inpatient drug utilization. Clinical guidelines recommend hospital-level management for severe Balamuthia cases.
The Clinic segment is anticipated to witness the fastest growth rate of 22.4% from 2026 to 2033, fueled by the expansion of outpatient follow-up, specialist infectious disease clinics, and telemedicine. Clinics enable early intervention and monitoring of oral therapy continuation. Hospital discharge programs increasingly rely on clinics for outpatient management. Reference-lab-linked clinics coordinate rapid diagnostic testing. The market sees growing adoption of clinics due to patient convenience and access. Clinics show the steepest percentage growth among end-users while hospitals remain dominant.
- By Distribution Channel
On the basis of distribution channel, the Balamuthia infection treatment market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The Hospital Pharmacy segment dominated the market with the largest market revenue share of 68.0% in 2025, driven by acute care, IV therapy, and special-access drug distribution. Hospitals often prioritize in-house pharmacy management for treatment regimens. The market also sees strong demand due to hospital procurement and parenteral compounding capabilities. Hospital pharmacy dominance is reinforced by tertiary care centers. Hospital pharmacies manage multi-drug and IV protocols efficiently. Acute case management centralizes distribution in hospitals.
The Online Pharmacy segment is anticipated to witness the fastest growth rate of 18.5% from 2026 to 2033, fueled by telemedicine, patient assistance programs, and home delivery of oral therapy. Online pharmacies improve accessibility to oral agents for discharged patients. Hospitals coordinate with online pharmacies for outpatient drug supply. The market sees strong adoption due to convenience, regulatory support for telehealth prescriptions, and continuity of therapy. Specialty online pharmacies expand access to rare-disease drugs. Online channels show the fastest percentage growth despite hospital pharmacy dominance.
Balamuthia Infection Treatment Market Regional Analysis
- North America dominated the Balamuthia infection treatment market with the largest revenue share of 42.3% in 2025, supported by advanced healthcare infrastructure, strong laboratory capabilities, and higher reported case identification
- Patients in the region have better access to specialized hospitals, reference laboratories, and multi-drug treatment protocols, enabling early diagnosis and initiation of combination therapy, which increases overall treatment volumes
- This widespread adoption is further supported by robust healthcare spending, advanced hospital pharmacy networks, and the availability of compassionate-use and investigational drugs, establishing North America as a leader in both clinical management and market revenue
U.S. Balamuthia Infection Treatment Market Insight
The U.S. Balamuthia infection treatment market captured the largest revenue share of 80% in 2025 within North America, fueled by the presence of advanced healthcare infrastructure and widespread adoption of molecular diagnostics. Clinicians are increasingly prioritizing rapid identification and early initiation of combination therapy for better survival outcomes. The growing number of tertiary care and infectious disease centers, combined with robust hospital pharmacy networks, further propels the market. Moreover, availability of compassionate-use and investigational drugs for rare CNS infections is significantly contributing to market expansion.
Europe Balamuthia Infection Treatment Market Insight
The Europe Balamuthia infection treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by strong healthcare systems, specialized hospitals, and growing investment in infectious disease research. The increase in awareness among clinicians, coupled with the demand for early diagnosis and multi-drug treatment regimens, is fostering market adoption. European hospitals are increasingly integrating advanced diagnostics such as PCR and biopsy, enabling timely therapeutic interventions. The region is experiencing significant growth across both inpatient and outpatient treatment settings, with hospitals and reference laboratories being key market drivers.
U.K. Balamuthia Infection Treatment Market Insight
The U.K. Balamuthia infection treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by heightened awareness of rare CNS infections and a focus on early diagnosis. Concerns regarding delayed identification and severe clinical outcomes are encouraging hospitals and specialty clinics to adopt advanced diagnostic tools and multi-drug regimens. In addition, the U.K.’s strong healthcare infrastructure and national infectious disease networks are expected to continue supporting market growth. Reference laboratories and tertiary hospitals remain central to treatment adoption.
Germany Balamuthia Infection Treatment Market Insight
The Germany Balamuthia infection treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced diagnostic facilities and high standards of clinical care. Hospitals and academic centers are increasingly adopting PCR, biopsy, and combination therapy protocols for Balamuthia infections. Germany’s focus on innovation in rare disease management and research contributes to market growth. The integration of molecular diagnostics and hospital pharmacy capabilities enhances treatment availability. Strong emphasis on patient safety, treatment efficacy, and rapid access to therapy promotes market adoption.
Asia-Pacific Balamuthia Infection Treatment Market Insight
The Asia-Pacific Balamuthia infection treatment market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by increasing awareness of rare CNS infections, expanding healthcare infrastructure, and rising government initiatives for infectious disease control. The region's growing number of tertiary hospitals, specialized clinics, and reference laboratories is driving adoption of advanced diagnostic and treatment protocols. Furthermore, as APAC countries improve access to oral and parenteral therapies, affordability and availability of treatment options are expanding to a wider patient population.
Japan Balamuthia Infection Treatment Market Insight
The Japan Balamuthia infection treatment market is gaining momentum due to the country’s high standard of healthcare, advanced molecular diagnostics, and focus on rare CNS infection management. Hospitals and specialized infectious disease centers are increasingly adopting multi-drug regimens and rapid diagnostic tests. The integration of hospital pharmacy services with referral networks is supporting timely initiation of therapy. In addition, Japan’s aging population increases demand for accessible and effective treatment options in both inpatient and outpatient settings, further propelling market growth.
India Balamuthia Infection Treatment Market Insight
The India Balamuthia infection treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to the country’s expanding tertiary care network, growing awareness among clinicians, and rising investments in diagnostic facilities. India is witnessing an increasing number of reported Balamuthia cases, prompting hospitals and specialized clinics to adopt combination therapy protocols. The push towards improving healthcare access in urban and semi-urban regions, along with the availability of oral and injectable therapy options, are key factors propelling market growth in India.
Balamuthia Infection Treatment Market Share
The Balamuthia Infection Treatment industry is primarily led by well-established companies, including:
- Gilead Sciences, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd (India)
- Bristol‑Myers Squibb Company (U.S.)
- Astellas Pharma (Japan)
- Xellia Pharmaceuticals A/S (Denmark)
- Cipla (India)
- Teva Pharmaceutical Industries Ltd (Israel)
- Dr. Reddy’s Laboratories Ltd (India)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- Amgen Inc. (U.S.)
- Hikma Pharmaceuticals plc (U.K.)
- Fresenius Kabi AG (Germany)
- Cadila Pharmaceuticals Ltd (India)
- Bharat Serums and Vaccines Ltd (India)
- Lupin (India)
- C.H. Boehringer Sohn AG & Co. KG (Germany)
What are the Recent Developments in Global Balamuthia Infection Treatment Market?
- In February 2024, researchers reported the first confirmed case of Balamuthia mandrillaris CNS infection in Pakistan, diagnosed via PCR and Sanger sequencing, which highlighted rising global awareness and the need for enhanced diagnostic vigilance in regions previously under‑recognized
- In October 2023, another research group published a study on zinc-oxide nanoparticle conjugates combined with existing antimicrobials (such as amphotericin B and antibiotics) that showed significant amoebicidal activity against Balamuthia in vitro while maintaining limited toxicity to human cells. This opens another promising line of investigation for novel and safer therapeutic delivery
- In April 2023, a study demonstrated that nanocarrier‑drug conjugates (nanoparticles) loaded with amphotericin B and curcumin showed enhanced anti-Balamuthia activity in vitro. Notably, the nanoparticle form of amphotericin B was more effective than the free drug, and the nanocarriers reduced drug toxicity against human cells in lab tests
- In January 2023, researchers in California reported a rare survival from Balamuthia mandrillaris granulomatous amebic encephalitis (GAE) using the repurposed drug nitroxoline, typically used for urinary tract infections. This is a landmark because Balamuthia encephalitis has historically had a > 90% mortality rate, and the case demonstrated that nitroxoline had strong in vitro amebicidal activity and was well tolerated in the patient
- In May 2022, Chinese clinicians published a report of four cutaneous Balamuthia mandrillaris cases treated with diminazene aceturate (a veterinary antiparasitic). In this case series, one patient was cured by monotherapy, two by a combination of excision + diminazene, and one died due to liver toxicity. It’s the first documented human application of this drug for Balamuthia skin infections
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.