Global Becker Muscular Dystrophy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.92 Billion
USD
2.83 Billion
2024
2032
USD
1.92 Billion
USD
2.83 Billion
2024
2032
| 2025 –2032 | |
| USD 1.92 Billion | |
| USD 2.83 Billion | |
|
|
|
|
Becker Muscular Dystrophy Treatment Market Size
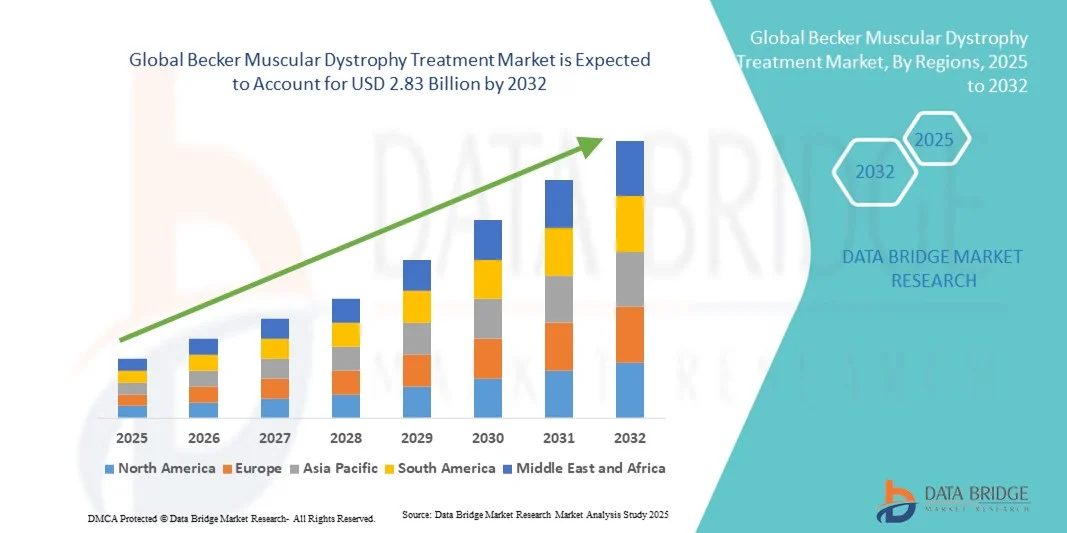
- The global becker muscular dystrophy treatment market size was valued at USD 1.92 billion in 2024 and is expected to reach USD 2.83 billion by 2032, at a CAGR of 5.00% during the forecast period
- The market growth is largely fueled by the rising prevalence of genetic neuromuscular disorders and the increasing focus on developing targeted therapies and gene-based treatments, leading to enhanced diagnosis and disease management globally
- Furthermore, growing research collaborations between pharmaceutical companies and academic institutions, along with government initiatives supporting rare disease treatment, are accelerating the uptake of becker muscular dystrophy Treatment solutions, thereby significantly boosting the industry's growth
Becker Muscular Dystrophy Treatment Market Analysis
- Becker Muscular Dystrophy (BMD) treatments, encompassing gene therapies, corticosteroids, and emerging molecular therapies, are becoming increasingly vital components of modern neuromuscular disease management due to their potential to improve muscle strength, slow disease progression, and enhance patients’ quality of life
- The escalating demand for Becker Muscular Dystrophy treatment is primarily driven by rising awareness of genetic disorders, growing investment in rare disease research, and increasing availability of advanced diagnostic technologies enabling early detection
- North America dominated the becker muscular dystrophy treatment market with the largest revenue share of 42.8% in 2024, characterized by a robust biotechnology sector, early adoption of novel gene-based therapies, and strong government and private funding for neuromuscular research. The U.S. continues to lead the region with expanding clinical trials and FDA approvals for innovative treatments
- Asia-Pacific is expected to be the fastest-growing region in the becker muscular dystrophy treatment market during the forecast period, registering a CAGR from 2025 to 2032, driven by increasing healthcare expenditure, improved access to genetic testing, and strategic collaborations among research institutions in countries such as Japan, China, and India
- The genetic testing segment dominated the largest market revenue share of 41.3% in 2024, driven by its unparalleled precision in identifying dystrophin gene mutations responsible for the disorder
Report Scope and Becker Muscular Dystrophy Treatment Market Segmentation
|
Attributes |
Becker Muscular Dystrophy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Becker Muscular Dystrophy Treatment Market Trends
Expansion of Gene Therapy Clinical Trials and Regulatory Advancements
- A significant and accelerating trend in the global becker muscular dystrophy (BMD) treatment Market is the ongoing expansion of gene therapy clinical trials and increasing regulatory support for innovative treatment modalities
- For instance, in June 2023, Sarepta Therapeutics, Inc. achieved FDA accelerated approval for its gene therapy Elevidys (delandistrogene moxeparvovec-rokl), marking a major step in advancing gene replacement strategies targeting dystrophin deficiencies associated with BMD and Duchenne Muscular Dystrophy
- Similarly, companies such as Pfizer, Solid Biosciences, and Regenxbio are conducting multiple late-stage clinical trials evaluating AAV-based therapies aimed at restoring or stabilizing muscle function, expanding treatment options for patients with Becker Muscular Dystrophy
- The global surge in clinical research initiatives is being supported by collaborative frameworks between pharmaceutical companies, research institutions, and government agencies focused on accelerating rare disease drug development
- Furthermore, increased adoption of next-generation sequencing (NGS) and biomarker-based patient selection is optimizing trial efficiency and improving success rates in gene therapy research
- Regulatory agencies including the U.S. FDA and EMA are implementing fast-track designations, breakthrough therapy approvals, and orphan drug incentives to promote faster commercialization of novel BMD treatments
- The trend is also characterized by greater transparency and global data sharing through registries such as the Treat-NMD Neuromuscular Network, enabling better clinical coordination and post-approval monitoring
- As a result, the Becker Muscular Dystrophy treatment landscape is evolving from symptom management to disease modification, driven by rapid advancements in genomic medicine
- The rising focus on long-term efficacy studies and patient-centric trial designs is fostering innovation in both gene therapy and molecular correction techniques
- In addition, partnerships between biotech companies and non-profit organizations are playing a vital role in funding early-stage clinical programs and expanding patient outreach
- This ongoing transformation is expected to enhance therapeutic diversity and drive strong revenue growth for the global Becker Muscular Dystrophy treatment market throughout the forecast period
- The market is therefore witnessing a fundamental shift toward more effective, targeted, and durable therapeutic solutions, supported by robust R&D and favorable regulatory momentum
Becker Muscular Dystrophy Treatment Market Dynamics
Driver
Rising Demand for Advanced Gene and Cell Therapies
- A major driving factor in the global Becker Muscular Dystrophy (BMD) treatment market is the rapid advancement and increasing adoption of innovative gene and cell therapy approaches aimed at targeting the underlying genetic mutations responsible for the disease
- For instance, in June 2023, Sarepta Therapeutics, Inc. received accelerated FDA approval for its gene therapy Elevidys (delandistrogene moxeparvovec-rokl) for Duchenne and Becker Muscular Dystrophy, marking a significant milestone in the expansion of precision-based treatments for rare neuromuscular disorders
- This approval, along with the growing pipeline of AAV-based and exon-skipping therapies, is reshaping the treatment landscape by offering more personalized and potentially curative options
- Pharmaceutical and biotech companies are increasingly investing in gene-editing technologies such as CRISPR and antisense oligonucleotides (ASOs) to address disease progression more effectively than conventional corticosteroid therapy
- Moreover, advancements in diagnostic techniques, including next-generation sequencing (NGS), are facilitating early disease detection and patient stratification, thereby enabling timely therapeutic intervention and improving overall prognosis
- The rising prevalence of genetic testing programs, coupled with growing patient awareness and support from advocacy organizations, further enhances treatment accessibility and accelerates research collaboration
- In addition, strong regulatory incentives such as orphan drug designations, fast-track approvals, and funding for rare diseases are driving global interest and investment in Becker Muscular Dystrophy treatment innovation
- The emergence of advanced therapeutic modalities, increased R&D funding, and expanding clinical trials across North America, Europe, and Asia-Pacific are collectively contributing to market expansion
- Increasing collaboration between biotech firms and research institutions is expected to result in more efficient therapy development and wider adoption of disease-modifying treatments
- The integration of digital health technologies for patient monitoring and therapy response tracking is further enhancing the management of Becker Muscular Dystrophy
- As a result, the Becker Muscular Dystrophy treatment market is anticipated to witness strong growth throughout the forecast period, supported by rapid biotechnological progress and favorable regulatory frameworks
Restraint/Challenge
High Treatment Costs and Limited Accessibility to Advanced Therapies
- Despite promising advancements in becker muscular dystrophy management, high therapy costs and limited global accessibility to gene-based treatments remain major restraints to market growth
- For instance, recently approved or pipeline gene therapies such as Elevidys can cost upwards of several million dollars per treatment, posing affordability challenges for patients and healthcare systems, particularly in developing economies
- The complexity of manufacturing viral vectors and ensuring large-scale production capacity further contributes to high costs and limited availability
- In addition, reimbursement limitations and variations in healthcare infrastructure across regions restrict patient access to innovative therapies, despite demonstrated efficacy in clinical trials
- While ongoing policy initiatives and funding programs are attempting to improve access to rare disease treatments, challenges persist in terms of equitable distribution and sustainable pricing models
- Furthermore, logistical and ethical challenges associated with gene therapy—such as long-term safety monitoring, immune response risks, and stringent regulatory requirements—can delay widespread clinical adoption
- Clinical trial enrollment difficulties, stemming from the rarity of the condition and the limited patient pool, often hinder the pace of research and approval timelines
- Addressing these challenges requires coordinated efforts between governments, payers, and biopharmaceutical companies to develop flexible reimbursement strategies and global access programs
- Increasing public-private partnerships focused on rare diseases and expanding patient registries can also help in streamlining research and broadening clinical access
- Moreover, the need for long-term data on safety, durability of effect, and real-world outcomes continues to be a critical consideration for regulatory agencies
- To ensure sustained market growth, manufacturers must focus on cost optimization, scalable production, and innovative financing solutions such as outcome-based pricing
Becker Muscular Dystrophy Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, and end user.
- By Treatment
On the basis of treatment, the Becker Muscular Dystrophy Treatment market is segmented into drugs, corticosteroids, angiotensin-converting enzyme (ACE) inhibitors, physical therapy, exercise, braces, mobility aids, breathing assistance, molecular therapy, gene replacement therapy, stem cell research, protein production alteration, and myoblast transplantation. The drugs segment dominated the largest market share of 35.8% in 2024, driven by their crucial role in symptom management and slowing disease progression. Corticosteroids, such as prednisone and deflazacort, remain first-line pharmacological options proven to enhance muscle strength and delay functional decline. The availability of generic formulations and the growing physician preference for evidence-based pharmacotherapy further strengthen this segment’s dominance. Rising collaborations among pharmaceutical firms and research institutes to develop more effective molecules addressing inflammation and muscle degeneration also contribute significantly. Moreover, the expanding patient pool, coupled with government reimbursement for standard pharmacological treatments, drives sustained market adoption across both developed and developing regions. Increasing awareness campaigns and regulatory approvals for novel therapeutic drugs are also likely to keep this segment leading throughout the forecast period.
The gene replacement therapy segment is anticipated to witness the fastest growth rate of CAGR 22.5% from 2025 to 2032, propelled by breakthroughs in genetic engineering, viral vector technologies, and precision medicine. This therapy aims to restore dystrophin protein production by delivering functional gene copies, addressing the root cause of the disease. Several biotech companies are advancing clinical trials for next-generation gene therapies demonstrating durable efficacy. Growing regulatory support, orphan drug designations, and high R&D investments are fueling rapid expansion. The segment’s potential to offer long-term functional benefits with one-time interventions is revolutionizing treatment paradigms, making it the fastest-growing area in the global Becker Muscular Dystrophy Treatment market.
- By Diagnosis
On the basis of diagnosis, the Becker Muscular Dystrophy Treatment market is segmented into enzyme assay, genetic testing, electromyography, and biopsy. The genetic testing segment dominated the largest market revenue share of 41.3% in 2024, driven by its unparalleled precision in identifying dystrophin gene mutations responsible for the disorder. With advances in next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies, genetic testing has become faster, more accessible, and cost-effective. The increasing adoption of early screening programs and family-based genetic counseling further reinforces this dominance. Moreover, government-supported newborn screening initiatives and greater awareness of hereditary muscular dystrophies are driving demand. Leading diagnostic companies are expanding test panels to include comprehensive genetic markers for muscular dystrophies, facilitating personalized treatment strategies. The segment’s clinical reliability and integration into therapeutic decision-making are establishing it as the standard diagnostic method worldwide.
The enzyme assay segment is projected to witness the fastest growth rate of CAGR 19.4% from 2025 to 2032, owing to advancements in biochemical analysis and the growing role of creatine kinase (CK) enzyme testing as a preliminary diagnostic step. Enzyme assays provide quick, non-invasive results that guide further molecular testing. Increasing adoption in hospitals and diagnostic laboratories for initial screening and disease monitoring contributes to its strong growth. The affordability and simplicity of enzyme-based testing make it particularly suitable in low-resource healthcare settings, supporting broad accessibility and higher utilization rates.
- By End User
On the basis of end user, the Becker Muscular Dystrophy Treatment market is segmented into hospitals, home care settings, clinics, and others. The hospitals segment accounted for the largest market revenue share of 48.6% in 2024, driven by the concentration of specialized neuromuscular care facilities and access to multidisciplinary treatment teams. Hospitals offer integrated diagnosis, medication management, physiotherapy, and respiratory support, ensuring comprehensive patient care. Increasing hospital-based clinical trials and the presence of advanced infrastructure for genetic and molecular therapies further reinforce this dominance. In addition, collaborations between hospitals and pharmaceutical companies for new drug evaluations are expanding patient access to novel treatments. Government investments in hospital-based research centers and growing awareness about muscular dystrophy care in tertiary facilities continue to bolster segment growth. Hospitals remain the primary centers for disease management, monitoring, and emergency interventions, supporting their leading market position.
The home care settings segment is expected to witness the fastest CAGR of 20.7% from 2025 to 2032, driven by the rising preference for patient-centric care models and the expansion of telemedicine platforms. Home-based treatment allows for greater comfort, cost savings, and continuous monitoring through connected medical devices. Families increasingly opt for home care due to accessibility, convenience, and the availability of remote physiotherapy and mobility assistance services. Moreover, the growing adoption of wearable health monitoring devices and government support for home rehabilitation programs are enhancing the feasibility of home-based management. Technological advancements, including smart mobility aids and telehealth consultations, are accelerating the adoption of home care settings, making it the fastest-growing end-user segment in the Becker Muscular Dystrophy Treatment market.
Becker Muscular Dystrophy Treatment Market Regional Analysis
- North America dominated the becker muscular dystrophy treatment market with the largest revenue share of 42.8% in 2024, characterized by a robust biotechnology sector, early adoption of novel gene-based therapies, and strong government and private funding for neuromuscular research
- The region’s dominance is further reinforced by the presence of leading biopharmaceutical companies such as Pfizer, Sarepta Therapeutics, and Solid Biosciences, which are actively conducting advanced clinical trials
- High awareness about genetic disorders and the increasing prevalence of Becker Muscular Dystrophy across the U.S. and Canada have stimulated diagnostic testing and patient identification. The U.S. continues to lead the regional landscape with rapid FDA approvals for innovative gene and molecular therapies and expanding reimbursement coverage for rare disease treatments
U.S. Becker Muscular Dystrophy Treatment Market Insight
The U.S. becker muscular dystrophy treatment market captured the largest revenue share in 2024 within North America, fueled by early access to advanced therapeutics, strong research funding, and increasing enrollment in clinical trials. Major pharmaceutical and biotech firms are investing heavily in genetic therapy pipelines to target disease-causing mutations. The growing prevalence of neuromuscular disorders, alongside initiatives by the Muscular Dystrophy Association (MDA), has significantly improved diagnosis and treatment awareness. The U.S. market also benefits from the accelerated approval pathways offered by the FDA for rare diseases. Increasing adoption of precision diagnostics, combined with growing healthcare expenditure, ensures continued market growth.
Europe Becker Muscular Dystrophy Treatment Market Insight
The Europe becker muscular dystrophy treatment market is projected to expand at a substantial CAGR during the forecast period, supported by a strong regulatory framework, improved access to orphan drugs, and growing investment in rare disease research. European healthcare systems are increasingly integrating gene and molecular therapies through national reimbursement programs, particularly in countries such as Germany, France, and the U.K. The surge in clinical research partnerships and EU-funded studies focusing on muscular dystrophy is also driving innovation. Rising awareness about early diagnosis, coupled with genetic screening initiatives, enhances treatment adoption. The availability of specialized neuromuscular centers across Europe, focusing on multidisciplinary care, further contributes to the region’s market expansion. The region’s focus on patient-centric therapeutic programs and advancements in biotechnology ensure its continued importance in the global Becker Muscular Dystrophy Treatment market.
U.K. Becker Muscular Dystrophy Treatment Market Insight
The U.K. becker muscular dystrophy treatment market is expected to grow at a noteworthy CAGR during the forecast period, driven by the increasing implementation of precision medicine programs and collaborations between the National Health Service (NHS) and biotech companies. A rise in genetic testing adoption and the government’s support for rare disease research are fueling market growth. The U.K.’s growing investment in translational medicine and clinical trial infrastructure is further promoting accessibility to advanced therapies.
Germany Becker Muscular Dystrophy Treatment Market Insight
The Germany becker muscular dystrophy treatment market is anticipated to expand at a considerable CAGR through 2032, propelled by technological innovations and strong institutional support for biomedical research. Germany’s established pharmaceutical industry and emphasis on quality clinical care underpin its leading role in Europe. Increasing collaborations with international research consortia and the adoption of novel molecular therapies are driving growth.
Asia-Pacific Becker Muscular Dystrophy Treatment Market Insight
The Asia-Pacific Becker Muscular Dystrophy Treatment market is expected to be the fastest-growing region during the forecast period, registering a CAGR from 2025 to 2032, driven by increasing healthcare expenditure, improved access to genetic testing, and strategic collaborations among research institutions in countries such as Japan, China, and India. Rapid urbanization, rising awareness about rare genetic disorders, and supportive government healthcare policies are promoting diagnosis and early intervention. The emergence of regional biopharmaceutical firms focusing on gene therapy development and clinical research is accelerating treatment accessibility. Japan and China are leading the regional growth with investments in cell and gene therapy platforms and patient registry programs. Enhanced healthcare infrastructure and the adoption of advanced molecular diagnostics further strengthen Asia-Pacific’s position as the fastest-growing market.
Japan Becker Muscular Dystrophy Treatment Market Insight
The Japan becker muscular dystrophy treatment market is gaining momentum due to strong government support for rare disease management and a rapidly expanding clinical trial ecosystem. Japan’s technological leadership and early adoption of genetic therapies are driving growth. Collaborations between universities and pharmaceutical companies are advancing local innovation in molecular and gene replacement therapies.
China Becker Muscular Dystrophy Treatment Market Insight
The China becker muscular dystrophy treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, supported by strong domestic manufacturing capabilities and expanding access to advanced therapies. The government’s focus on rare disease research and increasing investment in biotechnology are driving expansion. Widening availability of genetic screening and rising public health expenditure continue to strengthen China’s dominance within the regional market.
Becker Muscular Dystrophy Treatment Market Share
The Becker Muscular Dystrophy Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Santhera Pharmaceuticals (Switzerland)
- Catalyst Pharmaceuticals (U.S.)
- Capricor Therapeutics, Inc. (U.S.)
- Wave Life Sciences Ltd. (Singapore)
- Dyne Therapeutics, Inc. (U.S.)
- Edgewise Therapeutics, Inc. (U.S.)
- Genethon (France)
- Exonics Therapeutics (U.S.)
- Solid Biosciences Inc. (U.S.)
- ReveraGen BioPharma, Inc. (U.S.)
- Roche Holding AG (Switzerland)
- Nippon Shinyaku Co., Ltd. (Japan)
Latest Developments in Global Becker Muscular Dystrophy Treatment Market
- In June 2023, the U.S. Food & Drug Administration approved ELEVIDYS (delandistrogene moxeparvovec-rokl), the first gene-replacement therapy for certain pediatric patients with dystrophin deficiency, marking a landmark regulatory milestone that validated AAV-delivered micro/mini-dystrophin approaches and accelerated investment and clinical activity across the muscular-dystrophy field
- In June 2024, Pfizer announced topline results from its Phase-3 program for its mini/micro-dystrophin gene therapy showing the trial missed its primary motor-function endpoint and subsequently discontinued the program, a high-profile setback that prompted the industry to re-examine trial design, endpoints, dosing strategies and safety monitoring for systemic gene therapies in muscular dystrophies.
- In November 2024, REGENXBIO advanced its RGX-202 microdystrophin program through dose escalation and into the pivotal phase, dosing the first pivotal/Phase-3 patients and reporting encouraging expression data from earlier cohorts — developments that underscored continued momentum for alternative AAV microdystrophin constructs despite mixed results elsewhere
- In February 2025, Solid Biosciences reported positive interim microdystrophin expression results from early participants in its clinical program, showing meaningful protein expression in treated patients and supporting further clinical evaluation of its gene-therapy approach for dystrophinopathies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.