Global Biomarkers Market
Market Size in USD Billion
CAGR :
% 
 USD
56.94 Billion
USD
172.97 Billion
2024
2032
USD
56.94 Billion
USD
172.97 Billion
2024
2032
| 2025 –2032 | |
| USD 56.94 Billion | |
| USD 172.97 Billion | |
|
|
|
|
Biomarkers Market Size
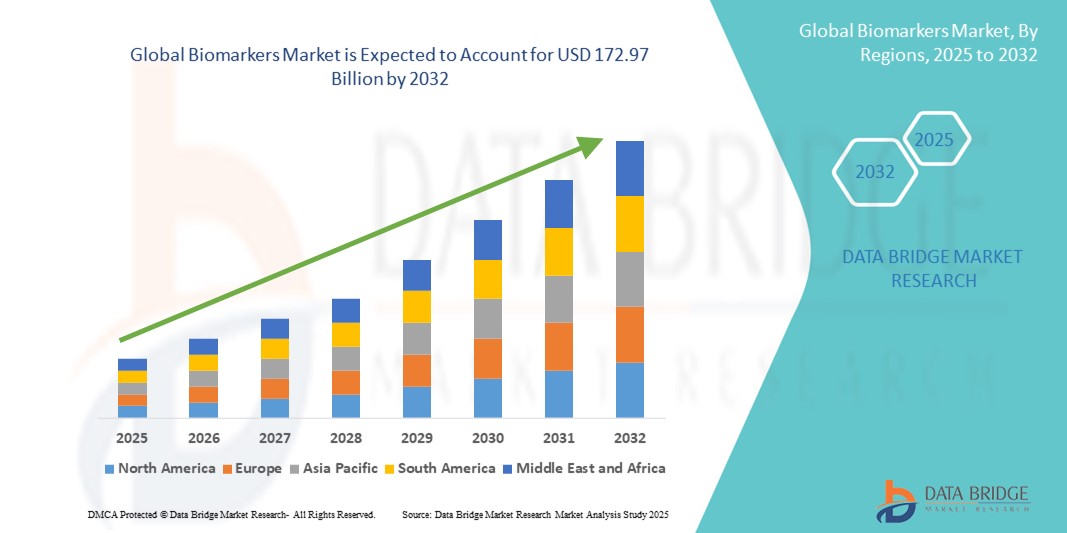
- The global biomarkers market size was valued at USD 56.94 billion in 2024 and is expected to reach USD 172.97 billion by 2032, at a CAGR of 14.90% during the forecast period
- The market growth is primarily driven by the increasing adoption of precision medicine and personalized healthcare approaches, which rely heavily on biomarkers for early diagnosis, disease monitoring, and treatment optimization
- Furthermore, rising investment in biotechnology and pharmaceutical research, coupled with advancements in genomics, proteomics, and metabolomics, is accelerating the development and application of biomarker-based solutions. These converging factors are significantly boosting the growth of the global biomarkers market, enabling improved patient outcomes, more targeted therapies, and enhanced clinical decision-making across healthcare sectors
Biomarkers Market Analysis
- Biomarkers, used as measurable indicators of biological processes, disease progression, or therapeutic response, are increasingly critical in diagnostics, drug development, and personalized medicine across oncology, cardiovascular, and neurological diseases
- The rising demand for biomarkers is primarily driven by increasing prevalence of chronic diseases, growing focus on precision medicine, advancements in high-throughput screening technologies, and the need for early and accurate disease detection
- North America dominated the biomarkers market with the largest revenue share of 41.55% in 2024, supported by advanced healthcare infrastructure, high patient awareness, and the presence of leading biotech and diagnostic companies. The U.S. specifically is witnessing rapid growth in biomarker discovery, validation, and clinical applications, aided by substantial investment in R&D, regulatory support, and adoption of AI-enabled biomarker analysis platforms
- Asia-Pacific is expected to be the fastest-growing region in the biomarkers market during the forecast period, with a projected CAGR of 23% from 2025 to 2032. Growth is fueled by increasing healthcare access, government initiatives promoting precision medicine, rising chronic disease burden, and the expansion of diagnostic laboratories in countries such as China, India, and Japan
- Cancer segment dominated the biomarkers market a revenue share of 46% in 2024, owing to its high prevalence and the critical role of biomarkers in early detection, prognosis, treatment monitoring, and precision oncology
Report Scope and Biomarkers Market Segmentation
|
Attributes |
Biomarkers Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Biomarkers Market Trends
Enhanced Convenience Through Technological Advancements
- A significant and accelerating trend in the global biomarkers market is the rapid adoption of advanced technologies in diagnostics, personalized medicine, and disease monitoring. These innovations are enhancing the accuracy, speed, and reliability of biomarker detection, enabling more precise and timely clinical decision-making
- For instance, recent developments in high-throughput sequencing, proteomics, and metabolomics have allowed researchers and clinicians to identify novel biomarkers for early disease detection, prognosis, and therapeutic response monitoring. Similarly, multi-omics integration platforms are providing comprehensive insights into disease mechanisms and patient stratification, offering a discreet and targeted approach in biomarker research
- Advanced data analytics and bioinformatics tools are increasingly being employed to analyze complex biological datasets, improving the predictive power of biomarkers and supporting the development of personalized treatment plans. Furthermore, integration with electronic health records and cloud-based platforms allows for seamless sharing and interpretation of biomarker data, enhancing collaboration across healthcare institutions
- The convergence of cutting-edge laboratory techniques, computational methods, and clinical research is fundamentally reshaping expectations for biomarker discovery and utilization. Consequently, companies and research institutions are focusing on developing high-sensitivity assays, multiplex detection methods, and non-invasive testing solutions to accelerate biomarker adoption in routine clinical practice
- The demand for innovative biomarker solutions is growing rapidly across both diagnostic and therapeutic sectors, as healthcare providers and pharmaceutical companies increasingly prioritize early detection, precision medicine, and real-time patient monitoring
Biomarkers Market Dynamics
Driver
Growing Need Due to Rising Healthcare Awareness and Personalized Medicine
- The increasing prevalence of chronic diseases, cancer, and genetic disorders, coupled with rising healthcare awareness and the push toward personalized medicine, is a significant driver for the heightened demand for biomarkers
- For instance, in April 2024, several leading biotech companies announced advancements in multi-omics-based biomarker discovery, focusing on early disease detection and precision therapeutics. Such initiatives are expected to drive the Biomarkers market growth in the forecast period
- As healthcare providers and pharmaceutical companies emphasize early diagnosis and targeted treatment strategies, biomarkers are playing a critical role in patient stratification, therapy monitoring, and prognostic evaluation, offering significant improvements over traditional diagnostic methods
- Furthermore, the expanding integration of biomarkers into clinical trials, drug development, and companion diagnostic tools is making them an essential component of modern healthcare systems, supporting more effective and personalized treatment plans
- The growing focus on non-invasive diagnostic techniques, high-throughput screening methods, and advanced laboratory technologies, along with increasing government and private funding for biomarker research, is propelling market adoption. The trend toward data-driven healthcare solutions and the rising availability of validated biomarker panels further contribute to sustained market growth
Restraint/Challenge
Challenges in Standardization, High Costs, and Regulatory Compliance
- One of the primary challenges restraining the biomarkers market is the lack of universally accepted standards for biomarker validation, testing, and interpretation. This variability across laboratories and institutions can lead to inconsistent results, making healthcare providers cautious about widespread adoption
- The high costs associated with advanced biomarker assays, multi-omics platforms, and specialized laboratory equipment also limit accessibility, particularly in developing regions and smaller healthcare facilities. These financial barriers can delay implementation despite the clinical benefits offered by biomarkers
- Regulatory complexities further challenge market growth, as introducing new biomarkers into clinical practice requires rigorous approval processes. Adherence to evolving guidelines and compliance with local and international regulatory standards can be time-consuming and resource-intensive for both diagnostic and pharmaceutical companies
- In addition, the need for highly skilled personnel to perform and interpret biomarker tests adds another layer of difficulty for adoption, especially in regions with limited trained workforce in genomics and molecular diagnostics
- While technological advancements are gradually improving accuracy and reducing costs, the perception of high expense and procedural complexity continues to limit broader utilization. Addressing these hurdles through standardized protocols, cost-effective solutions, enhanced training, and streamlined regulatory pathways is essential to ensure sustained growth in the Biomarkers market
Biomarkers Market Scope
The market is segmented on the basis of type, application, product type, technology, and indication.
- By Type
On the basis of type, the biomarkers market is segmented into safety, efficacy, and validation. The safety segment dominated the market with the largest revenue share of 42% in 2024, as safety biomarkers are crucial for detecting potential adverse reactions, monitoring patient safety, and ensuring the tolerability of drugs and therapeutic interventions across preclinical and clinical stages. These biomarkers are integral to regulatory submissions, toxicity studies, and post-market surveillance programs, helping reduce clinical risks and support safer drug development. They provide actionable insights that guide dosage adjustments, minimize adverse events, and facilitate compliance with global safety standards.
The Validation segment is expected to witness the fastest CAGR of 20% from 2025 to 2032, propelled by the increasing need for standardized, reproducible, and reliable biomarkers to support diagnostics, drug development, and translational research. Rising regulatory requirements, heightened emphasis on reproducibility in scientific studies, and increased investment in validation initiatives are further driving the growth of this segment, positioning it as a key focus area in the biomarkers market.
- By Application
On the basis of application, the biomarkers market is segmented into diagnostics development, drug discovery and development, personalized medicine, disease-risk assessment, and others. Diagnostics development dominated with a revenue share of 40% in 2024, as biomarkers are extensively employed for early disease detection, clinical diagnostics, and population-wide screening programs. Their ability to track disease onset, progression, and patient responses has made them indispensable in diagnostic research and clinical practice.
Personalized Medicine is expected to witness the fastest CAGR of 22% from 2025 to 2032, driven by the growing emphasis on precision medicine and targeted therapies that rely on predictive and prognostic biomarkers. The integration of biomarkers into companion diagnostics, individualized treatment planning, and patient-specific therapy decisions is fueling demand in this segment, enabling more accurate, effective, and personalized healthcare solutions while supporting long-term market expansion.
- By Product Type
On the basis of product type, the Biomarkers market is segmented into Consumables, Services, and Software. Consumables dominated the market with a revenue share of 45% in 2024, due to the continuous need for reagents, assay kits, sample preparation tools, and platforms used in biomarker research, clinical testing, and high-throughput screening applications. These consumables form the backbone of laboratory workflows, ensuring reliable experimental results and consistent performance in both research and clinical environments.
Software is expected to witness the fastest CAGR of 21% from 2025 to 2032, driven by the increasing adoption of bioinformatics, AI-driven analytics, and cloud-based platforms that facilitate comprehensive integration, interpretation, and visualization of biomarker data. The combination of advanced software solutions with consumables and services is enhancing research efficiency, improving data accuracy, and streamlining workflows, ultimately contributing to more informed decision-making and accelerated development in the Biomarkers sector.
- By Technology
On the basis of technology, the Biomarkers market is segmented into Safety Biomarkers, Efficacy Biomarkers, and Validation Biomarkers. Safety Biomarkers dominated with a revenue share of 43% in 2024, as they are extensively utilized to monitor toxicological effects, assess patient tolerability, and ensure overall safety during drug development and clinical trials. These biomarkers are critical for preclinical studies, helping researchers identify potential adverse effects before human testing, and they support continuous monitoring during clinical trials to maintain regulatory compliance. Safety biomarkers also play a pivotal role in post-market surveillance, minimizing risks associated with therapy administration and guiding dose adjustments.
Efficacy Biomarkers are expected to witness the fastest CAGR of 23% from 2025 to 2032. This rapid growth is driven by the increasing need to evaluate treatment response, measure therapeutic effectiveness, and perform outcome-based research, particularly in targeted therapies and personalized medicine. The integration of efficacy biomarkers in clinical trials, drug development programs, and regulatory frameworks is further enhancing their adoption and reinforcing their significance across the healthcare ecosystem.
- By Indication
On the basis of indication, the biomarkers market is segmented into cancer, cardiovascular disorders, neurological disorders, immunological disorders, and others. Cancer dominated with a revenue share of 46% in 2024, owing to its high prevalence and the critical role of biomarkers in early detection, prognosis, treatment monitoring, and precision oncology. Biomarkers help identify specific molecular targets, predict disease progression, and guide personalized therapy decisions, making them indispensable in oncology research and clinical practice.
Neurological disorders are expected to witness the fastest CAGR of 24% from 2025 to 2032. The rapid growth in this segment is driven by the rising incidence of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and other cognitive impairments. Increased focus on early diagnostic markers, advancements in understanding disease pathophysiology, and the development of targeted therapeutic strategies are supporting strong adoption of biomarkers in this area, fueling robust market expansion over the forecast period.
Biomarkers Market Regional Analysis
- North America dominated the biomarkers market with the largest revenue share of 41.55% in 2024, primarily supported by a well-established healthcare infrastructure, high patient awareness, and the presence of leading biotech and diagnostic companies
- The region benefits from extensive R&D investments, advanced laboratory facilities, and regulatory support that enable rapid adoption of novel biomarker technologies
- The increasing focus on personalized medicine, early disease detection, and treatment monitoring has significantly driven demand across hospitals, diagnostic centers, and research institutions
U.S. Biomarkers Market Insight
The U.S. biomarkers market captured the largest revenue share in North America in 2024, fueled by substantial growth in biomarker discovery, validation, and clinical applications. The market is supported by high adoption of AI-enabled biomarker analysis platforms, growing prevalence of chronic and oncologic diseases, and rising participation in clinical trials. Collaboration between biotech firms, research institutions, and hospitals is enhancing innovation, while increasing awareness among healthcare professionals and patients is driving utilization. These factors collectively contribute to the U.S. market’s robust expansion.
Europe Biomarkers Market Insight
The Europe biomarkers market is expected to witness steady growth throughout the forecast period, driven by strong healthcare systems, government initiatives promoting personalized medicine, and increasing investment in AI-powered biomarker technologies. Countries such as Germany, France, and the U.K. are adopting advanced diagnostic infrastructure to facilitate early detection, disease monitoring, and precision treatment. Emphasis on clinical accuracy, regulatory compliance, and integration of biomarkers into routine diagnostics is encouraging growth across hospitals, research centers, and specialized clinics.
U.K. Biomarkers Market Insight
The U.K. biomarkers market is anticipated to grow at a significant CAGR during the forecast period, fueled by increasing R&D activity, regulatory support for clinical trials, and growing adoption of biomarker-guided therapies. Rising awareness of personalized medicine, along with substantial investments by healthcare providers in advanced diagnostic solutions, is boosting market expansion. The integration of biomarkers in oncology, cardiovascular, and neurological diagnostics is creating new avenues for innovation and clinical application.
Germany Biomarkers Market Insight
The Germany biomarkers market is projected to expand steadily during the forecast period, driven by strong healthcare infrastructure, a robust biotechnology sector, and high patient awareness. Germany’s focus on innovation and the integration of AI-based diagnostic tools is enhancing biomarker applications in early detection, treatment planning, and monitoring of chronic and oncologic diseases. Increasing collaboration between research institutions and healthcare providers is further strengthening market growth.
Asia-Pacific Biomarkers Market Insight
The Asia-Pacific biomarkers market is expected to be the fastest-growing region during the forecast period, with a projected CAGR of 23% from 2025 to 2032. Growth is fueled by increasing access to healthcare services, rising prevalence of chronic diseases, government initiatives promoting precision medicine, and expansion of diagnostic laboratories in countries such as China, India, and Japan. Rising investments in biotechnology research, growing patient awareness, and the adoption of advanced biomarker platforms for disease diagnosis and treatment monitoring are key factors driving market expansion in the region.
Japan Biomarkers Market Insight
The Japan biomarkers market is gaining momentum due to the country’s advanced healthcare system, strong R&D capabilities, and growing demand for precision diagnostics. Clinical applications of biomarkers in oncology, cardiovascular, and neurological disorders are expanding rapidly, supported by government incentives, regulatory frameworks, and integration with AI-enabled diagnostic platforms. Increasing awareness among healthcare professionals and patients is further contributing to market growth.
China Biomarkers Market Insight
The China biomarkers market accounted for the largest revenue share in the Asia-Pacific region in 2024, driven by rapid urbanization, rising healthcare expenditures, expanding diagnostic infrastructure, and increasing patient awareness. The adoption of biomarkers is being fueled by government initiatives promoting biotechnology innovation, strong domestic manufacturers, and the growing focus on precision medicine and early disease detection. Increasing collaboration between hospitals, research institutions, and diagnostic companies is further accelerating market growth in China.
Biomarkers Market Share
The Biomarkers industry is primarily led by well-established companies, including:
- Enzo Biochem Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Merck KGaA (Germany)
- PerkinElmer (U.S.)
- QIAGEN (Germany)
- Agilent Technologies, Inc. (U.S.)
- Bruker (U.S.)
- Epigenomics AG (Germany)
- MESO SCALE DIAGNOSTICS, LLC (U.S.)
- EKF Diagnostics Holdings plc (U.K.)
- General Electric Company (U.S.)
- Nexus-Dx (U.S.)
- LifeSign LLC (U.S.)
- F.Hoffman-La Roche Ltd (Switzerland)
- Thermo Fischer Scientific Inc. (U.S.)
- Eurofins Scientific (Luxembourg)
- Abbott (U.S.)
- Charles River Laboratories International Inc. (U.S.)
- Sino Biological, Inc. (China)
- CENTOGENE N.V. (Germany)
Latest Developments in Global Biomarkers Market
- In April 2023, the U.S. National Institutes of Health (NIH) announced a USD4 million funding initiative to Eastern Virginia Medical School (EVMS) for the research and development of biomarkers aimed at the early detection of aggressive prostate cancer. This investment underscores the growing emphasis on precision diagnostics and personalized medicine in oncology
- In July 2025, Revelation Biosciences reported that its Phase 1 clinical study of the Gemini candidate successfully met the primary safety endpoint and demonstrated statistically significant biomarker activity. This milestone indicates promising potential for the treatment in triggering biological markers pivotal for efficacy in disease treatment
- In August 2025, Connecticut passed legislation requiring health insurance providers to cover biomarker testing starting January 1, 2026. This policy initiative aims to improve the diagnosis and treatment of various chronic diseases, including Alzheimer's, cancer, and Parkinson’s, by mandating coverage for biomarker tests under both individual and group insurance plans
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.