Global Biopharmaceuticals Manufacturing Consumables Testing Market
Market Size in USD Million
CAGR :
% 
 USD
714.47 Million
USD
1,800.84 Million
2025
2033
USD
714.47 Million
USD
1,800.84 Million
2025
2033
| 2026 –2033 | |
| USD 714.47 Million | |
| USD 1,800.84 Million | |
|
|
|
|
Biopharmaceuticals Manufacturing Consumables Testing Market Size
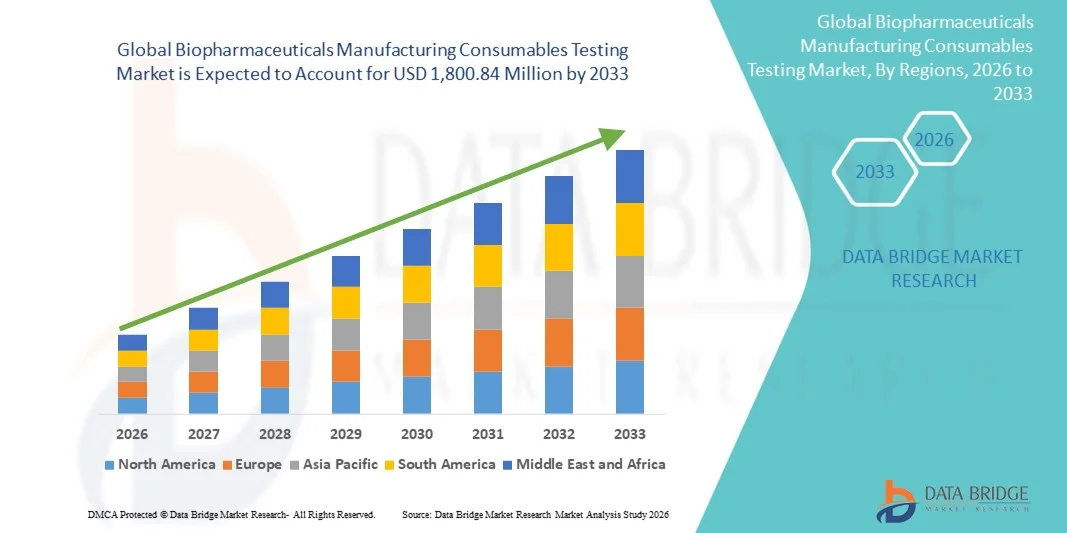
- The global biopharmaceuticals manufacturing consumables testing market size was valued at USD 714.47 million in 2025 and is expected to reach USD 1,800.84 million by 2033, at a CAGR of 12.25% during the forecast period
- The market growth is primarily driven by increasing biopharmaceutical production, stringent regulatory compliance requirements, and the critical need for high-quality consumables testing to ensure product safety, efficacy, and reliability
- In addition, rising investments in biologics, personalized medicines, and contract manufacturing organizations (CMOs) are expanding the demand for advanced consumables testing solutions, thereby accelerating the adoption of robust quality control measures and propelling market growth
Biopharmaceuticals Manufacturing Consumables Testing Market Analysis
- Biopharmaceuticals manufacturing consumables testing, including laboratory testing, custom testing, and compendial and multi-compendial laboratory testing, is becoming an essential component of biologics production, ensuring product safety, compliance, and process reliability across both small- and large-scale manufacturing facilities
- The growing demand for biopharmaceuticals, coupled with stringent regulatory requirements from agencies such as the FDA and EMA, is driving the adoption of advanced consumables testing services to minimize contamination risks and ensure batch-to-batch consistency
- North America dominated the biopharmaceuticals manufacturing consumables testing market with the largest revenue share of 39.4% in 2025, supported by a mature biopharma industry, significant R&D investments, and a robust presence of key service providers, particularly in the U.S., where innovation in laboratory testing and vendor qualification programs is rapidly advancing
- Asia-Pacific is expected to be the fastest growing region in the market during the forecast period due to the expansion of contract manufacturing organizations (CMOs), increasing biologics production, and rising regulatory focus on quality and safety in emerging markets such as China and India
- Laboratory testing segment dominated the market with a share of 42.6% in 2025, driven by its critical role in verifying the quality of formulation excipients, active pharmaceutical ingredients (APIs), and compendial methods (USP/EP/JP) based vendor qualification program support, ensuring compliance and product safety in modern biopharmaceutical manufacturing
Report Scope and Biopharmaceuticals Manufacturing Consumables Testing Market Segmentation
|
Attributes |
Biopharmaceuticals Manufacturing Consumables Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Biopharmaceuticals Manufacturing Consumables Testing Market Trends
Shift Towards Advanced Analytical and Custom Testing Services
- A significant and accelerating trend in the global biopharmaceuticals manufacturing consumables testing market is the increasing adoption of advanced analytical methods and custom testing services to ensure higher accuracy, reproducibility, and compliance with evolving regulatory standards
- For instance, companies such as SGS Life Sciences provide bespoke testing solutions for single-use systems, filtration units, and raw materials to meet specific client requirements, ensuring process integrity and product safety
- Integration of high-throughput testing platforms with automated analytics enables faster detection of impurities, contamination, or deviations in active pharmaceutical ingredients (APIs) and formulation excipients, reducing production delays and supporting consistent batch quality
- These advanced testing approaches facilitate centralized quality control, allowing manufacturers to monitor multiple consumables and raw material attributes through a single workflow, thereby optimizing production efficiency and compliance adherence
- This trend toward more precise, scalable, and customized testing solutions is reshaping manufacturer expectations for quality assurance and regulatory readiness. Consequently, service providers such as Eurofins are expanding AI-assisted testing platforms to enhance predictive analytics for raw material performance and vendor qualification programs
- The demand for sophisticated laboratory and custom testing services is growing rapidly across both contract manufacturing organizations (CMOs) and in-house biopharmaceutical production, as companies increasingly prioritize process reliability, regulatory compliance, and product safety
Biopharmaceuticals Manufacturing Consumables Testing Market Dynamics
Driver
Increasing Biopharmaceutical Production and Regulatory Oversight
- The rising global demand for biopharmaceuticals, along with stringent regulatory requirements from agencies such as the FDA and EMA, is a significant driver for the adoption of consumables testing services
- For instance, in March 2025, Charles River Laboratories expanded its testing capabilities for formulation excipients and APIs, targeting compliance with USP, EP, and JP standards to support biologics manufacturers
- As manufacturers aim to minimize contamination risks and ensure consistent product quality, consumables testing provides critical validation and monitoring of raw materials and single-use components
- Furthermore, the increasing use of contract manufacturing organizations (CMOs) and complex biologics production workflows necessitates reliable and scalable testing services to maintain batch-to-batch consistency and regulatory compliance
- The growing emphasis on high-quality biologics production, coupled with increased outsourcing of testing services, is making laboratory and custom testing an integral part of modern biopharmaceutical manufacturing processes
- Technological advancements in testing methodologies, such as rapid microbial detection and high-sensitivity assays, are enabling faster validation and boosting adoption across large-scale manufacturing facilities
Restraint/Challenge
High Costs and Specialized Equipment Requirement
- The high operational costs associated with advanced consumables testing and the need for specialized analytical equipment pose a significant challenge to market expansion
- For instance, the deployment of multi-compendial laboratory testing for APIs and excipients requires investment in high-precision instruments, skilled personnel, and ongoing calibration and maintenance
- Smaller manufacturers or CMOs in emerging markets may face barriers to adoption due to the substantial upfront and recurring costs involved, limiting market penetration in cost-sensitive regions
- In addition, strict adherence to multiple regulatory standards, including USP, EP, and JP, demands continuous training and validation, which can further increase operational complexity and expenses. Addressing these challenges through scalable testing solutions, technology-sharing models, and strategic partnerships will be crucial for expanding market reach while maintaining quality and compliance
- Limited availability of trained personnel with expertise in specialized testing techniques can slow down service adoption, particularly in emerging regions with growing biopharma manufacturing activity
- Volatility in raw material supply and variability in quality can introduce additional testing complexities and costs, challenging consistent service delivery and operational efficiency
Biopharmaceuticals Manufacturing Consumables Testing Market Scope
The market is segmented on the basis of service and raw material type.
- By Service
On the basis of service, the market is segmented into laboratory testing, custom testing / customer proprietary testing, and compendial and multi-compendial laboratory testing. The laboratory testing segment dominated the market with the largest revenue share of 42.6% in 2025, driven by its critical role in ensuring the quality, safety, and compliance of biopharmaceutical consumables. Routine laboratory testing validates formulation excipients, active pharmaceutical ingredients (APIs), and single-use systems to meet stringent regulatory requirements. It provides manufacturers with reliable data to maintain batch-to-batch consistency and prevent contamination. The segment benefits from high adoption among contract manufacturing organizations (CMOs) and large-scale biopharma facilities that prioritize robust quality control processes. Moreover, laboratory testing offers scalable solutions, allowing facilities to handle high volumes of samples with standardized procedures. Its widespread adoption is further fueled by regulatory mandates such as USP, EP, and JP compliance requirements, making it a cornerstone of modern biopharmaceutical production.
The custom testing / customer proprietary testing segment is anticipated to witness the fastest growth rate from 2026 to 2033, driven by the increasing demand for specialized and client-specific testing solutions. Manufacturers and CMOs are seeking tailored testing services for unique formulations, proprietary excipients, and emerging biologics. Custom testing ensures precise compliance with client specifications and regulatory expectations while enabling innovation in biopharmaceutical development. The growing complexity of biologics production, along with outsourcing trends in testing, is propelling the adoption of these services. Providers offering flexible, rapid, and scalable custom testing solutions are gaining significant traction. In addition, technological advancements in analytical instrumentation allow for more accurate and efficient custom testing, further accelerating market growth in this segment.
- By Raw Material Type
On the basis of raw material type, the market is segmented into formulation excipients, active pharmaceutical ingredients (API), and compendial methods (USP / EP / JP) based vendor qualification program support. The API testing segment dominated the market in 2025 due to the critical importance of verifying the purity, potency, and stability of active pharmaceutical ingredients before use in biologics manufacturing. Accurate API testing prevents contamination, ensures therapeutic efficacy, and supports regulatory compliance. The segment benefits from high adoption across all stages of drug development, from preclinical to commercial production. Manufacturers prioritize API testing to mitigate risks in biologics production and maintain quality standards across multiple batches. The presence of stringent regulatory frameworks worldwide further reinforces the dominance of this segment. Advanced analytical methods and automated platforms enhance the efficiency and reliability of API testing, contributing to its continued market leadership.
The compendial methods based vendor qualification program support segment is expected to witness the fastest growth rate from 2026 to 2033, driven by the increasing need for standardized testing protocols to qualify raw material vendors. Programs based on USP, EP, and JP guidelines help manufacturers ensure that all materials meet global quality standards. The growth of contract manufacturing and international supply chains is fueling demand for such programs to maintain compliance and reduce supply chain risks. In addition, regulatory authorities are emphasizing vendor qualification as part of GMP compliance, boosting adoption. Companies offering integrated vendor qualification and testing solutions, including multi-compendial support, are capturing new market opportunities. Advanced data management and reporting tools further enhance the efficiency and appeal of this growing segment.
Biopharmaceuticals Manufacturing Consumables Testing Market Regional Analysis
- North America dominated the biopharmaceuticals manufacturing consumables testing market with the largest revenue share of 39.4% in 2025, supported by a mature biopharma industry, significant R&D investments, and a robust presence of key service providers
- Manufacturers and CMOs in the region prioritize rigorous testing of formulation excipients, APIs, and single-use systems to ensure product safety, quality, and compliance with global standards
- This widespread adoption is further supported by high R&D investments, advanced analytical infrastructure, and the presence of leading testing service providers, establishing North America as the primary hub for high-quality and reliable consumables testing services
U.S. Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The U.S. biopharmaceuticals manufacturing consumables testing market captured the largest revenue share of 82% in 2025 within North America, fueled by the country’s well-established biopharmaceutical industry and strong regulatory oversight from the FDA. Manufacturers are increasingly prioritizing high-quality testing of formulation excipients, APIs, and single-use systems to ensure product safety, compliance, and process reliability. The growing adoption of contract manufacturing organizations (CMOs) and advanced biologics production workflows further propels market growth. In addition, the integration of automated and high-throughput testing technologies is enhancing efficiency and accuracy, supporting large-scale operations. Robust R&D investments, skilled workforce availability, and the presence of leading testing service providers further reinforce the U.S. as a key hub for consumables testing.
Europe Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The Europe biopharmaceuticals manufacturing consumables testing market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent regulatory requirements from the EMA and the growing demand for high-quality biologics production. Increasing urbanization and the expansion of CMOs in countries such as Germany, France, and Switzerland are fostering the adoption of advanced testing services. European manufacturers are also focusing on ensuring compliance with USP, EP, and JP compendial standards, enhancing the reliability of production processes. The region is witnessing significant growth across both small- and large-scale biologics facilities, with testing services being incorporated into new projects and expansions. In addition, government initiatives supporting pharmaceutical innovation and quality control further stimulate market development.
U.K. Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The U.K. biopharmaceuticals manufacturing consumables testing market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing demand for high-quality biologics and rigorous regulatory compliance. Rising awareness regarding safety, quality, and efficacy is encouraging manufacturers and CMOs to adopt advanced laboratory and custom testing services. The U.K.’s strong pharmaceutical research ecosystem, combined with robust contract testing services, is expected to continue stimulating market growth. Furthermore, the expansion of biologics production and the increasing complexity of formulations necessitate sophisticated consumables testing solutions to ensure consistent product quality and compliance.
Germany Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The Germany biopharmaceuticals manufacturing consumables testing market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s strong focus on innovation, high-quality manufacturing standards, and regulatory compliance. Germany’s mature pharmaceutical and biotechnology infrastructure promotes the adoption of laboratory, custom, and compendial testing services, particularly in large-scale biologics production. Integration of testing workflows with automated quality control systems is increasingly prevalent, enhancing efficiency and accuracy. Local manufacturers and CMOs also emphasize environmental sustainability and process optimization, driving the adoption of advanced testing methods.
Asia-Pacific Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The Asia-Pacific biopharmaceuticals manufacturing consumables testing market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by increasing biologics production, expanding CMOs, and rising regulatory focus in countries such as China, Japan, and India. The region’s growing pharmaceutical manufacturing capacity, combined with government initiatives promoting compliance and quality assurance, is driving the adoption of testing services. Moreover, the availability of cost-effective testing solutions and local service providers is making consumables testing more accessible across emerging markets. Technological advancements and the rising need for high-quality biologics are further contributing to market expansion.
Japan Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The Japan biopharmaceuticals manufacturing consumables testing market is gaining momentum due to the country’s advanced pharmaceutical research ecosystem, increasing biologics production, and stringent regulatory environment. Manufacturers are adopting high-precision laboratory and custom testing services to ensure compliance and product reliability. Integration of testing workflows with automated analytics and vendor qualification programs is enhancing operational efficiency. The demand for rigorous testing is also driven by Japan’s focus on quality, safety, and process standardization in both in-house and CMO-operated facilities. In addition, the adoption of innovative biologics and complex formulations supports the growth of specialized testing services.
India Biopharmaceuticals Manufacturing Consumables Testing Market Insight
The India biopharmaceuticals manufacturing consumables testing market accounted for the largest revenue share in Asia Pacific in 2025, attributed to the country’s expanding pharmaceutical manufacturing sector, rapid biologics production, and rising regulatory awareness. India is emerging as a key hub for CMOs and contract testing services, supporting both domestic and international clients. The push towards compliance with global quality standards, increasing investment in analytical infrastructure, and the availability of cost-effective testing solutions are driving adoption. In addition, the growing demand for high-quality biologics and personalized medicines is fueling the need for laboratory, custom, and compendial testing services in India.
Biopharmaceuticals Manufacturing Consumables Testing Market Share
The Biopharmaceuticals Manufacturing Consumables Testing industry is primarily led by well-established companies, including:
- Alcami Corporation (U.S.)
- Merck KGaA (Germany)
- Eurofins Scientific (Luxembourg)
- Agilent Technologies, Inc. (U.S.)
- Charles River Laboratories (U.S.)
- Catalent, Inc (U.S.)
- Element Materials Technology (U.K.)
- Pace Analytical Services, LLC (U.S.)
- Nelson Laboratories, LLC (U.S.)
- BioSpectra, Inc. (U.S.)
- Avomeen Analytical Services (U.S.)
- MabPlex International Ltd. (U.K.)
- SGS SA (Switzerland)
- Lonza (Switzerland)
- TOXIKON (U.S.)
- Boston Scientific Corporation (U.S.)
- STERIS (U.S.)
- Pharmetric Laboratory (U.K.)
- Albany Molecular Research Inc. (U.S.)
- Triclinic Labs, Inc. (U.S.)
What are the Recent Developments in Global Biopharmaceuticals Manufacturing Consumables Testing Market?
- In September 2024, Eurofins Scientific expanded its bio‑pharma product testing network in the U.S. through the acquisition of Infinity Laboratories, Inc. (eight labs in the U.S.), broadening its micro‑biology, chemistry and package testing footprint for biopharma consumables and thus strengthening capacity for consumables testing services
- In April 2024, Charles River Laboratories announced a launch of alternative testing methods to reduce reliance on animal testing in its biopharma and consumables testing services, reflecting a shift toward more advanced, ethical consumables testing workflows
- In February 2024, Eurofins announced a new “Platform Approach” for rapid GMP raw‑material testing (chemistry/biochemistry/microbiology/viral) covering high‑risk components at its Lancaster, PA raw materials laboratory, signifying enhanced offerings for raw‑material and consumables testing for biologics manufacturing
- In January 2024, Rapid Micro Biosystems filed its 10‑K, highlighting the availability of its automated consumables supporting microbiology QC workflows for biologics/consumables, illustrating increased innovation in consumables testing tooling
- In March 2022, Intertek Group plc announced the acquisition of SASTech LLC in order to expand its pharmaceutical & biopharmaceutical testing services bolstering capacity for consumables testing in the biopharma supply chain
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.