Global Blepharospasm Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
409.83 Million
USD
666.63 Million
2024
2032
USD
409.83 Million
USD
666.63 Million
2024
2032
| 2025 –2032 | |
| USD 409.83 Million | |
| USD 666.63 Million | |
|
|
|
|
Blepharospasm Treatment Market Size
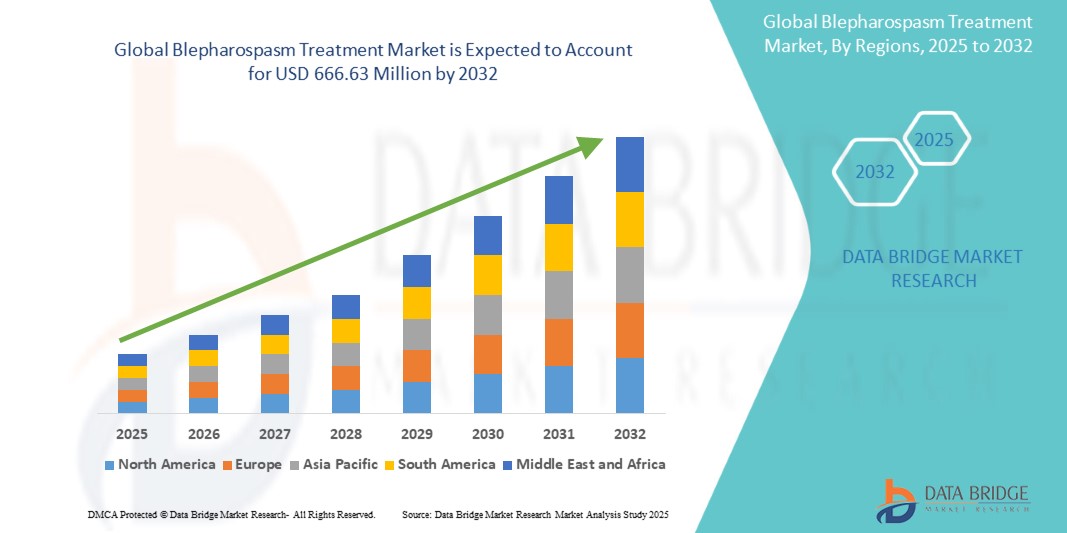
- The global blepharospasm treatment market size was valued at USD 409.83 Million in 2024 and is expected to reach USD 666.63 Million by 2032, at a CAGR of6.27% during the forecast period
- The market growth is largely fueled by the growing adoption of advanced neurological therapies and technological progress in drug delivery and surgical interventions, leading to improved diagnosis and management of blepharospasm in both clinical and outpatient settings
- Furthermore, rising patient demand for effective, minimally invasive, and long-lasting treatment options is establishing botulinum toxin injections and neuromodulation therapies as the standard of care. These converging factors are accelerating the uptake of blepharospasm treatment solutions, thereby significantly boosting the industry's growth
Blepharospasm Treatment Market Analysis
- Blepharospasm treatment, encompassing pharmacological, surgical, and device-based interventions, is increasingly gaining traction as a critical segment of the movement disorder therapeutics landscape, owing to the growing awareness, rising diagnosis rates, and advancements in botulinum toxin therapies
- The rising demand for effective blepharospasm treatment is primarily fueled by an aging population, increased prevalence of neurological disorders, greater access to specialty clinics, and expanding healthcare coverage in developing economies
- North America dominated the blepharospasm treatment market with the largest revenue share of 41.8% in 2024, attributed to early adoption of botulinum toxin therapies, availability of advanced healthcare infrastructure, and a strong presence of leading pharmaceutical and device manufacturers. The U.S. continues to lead regional growth due to increased diagnosis rates, high awareness among patients, and favorable reimbursement policies
- Asia-Pacific is expected to be the fastest growing region in the blepharospasm treatment market during the forecast period, with a projected CAGR of 8.5% from 2025 to 2032, driven by increasing urbanization, growing awareness about movement disorders, and improving access to neuro-ophthalmology services in countries such as China, India, and Japan
- The primary blepharospasm segment dominated the blepharospasm treatment market with a market share of 62.3% in 2024, owing to its higher prevalence as an idiopathic condition with no identifiable underlying disease, along with increasing awareness, improved clinical diagnosis, and a growing number of patients actively seeking treatment
Report Scope and Blepharospasm Treatment Market Segmentation
|
Attributes |
Blepharospasm Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Blepharospasm Treatment Market Trends
“Growing Shift Toward Personalized and Targeted Therapies”
- A significant and accelerating trend in the global blepharospasm treatment market is the advancement of personalized treatment options, including the development of tailored botulinum toxin regimens and alternative therapies based on patient-specific response profiles. This approach is significantly enhancing clinical outcomes and improving patient satisfaction
- For instance, neurologists are increasingly adjusting injection sites and dosages of botulinum toxin based on facial muscle mapping and patient-reported symptom severity, leading to more precise and effective treatment plans. Similarly, the use of adjunct therapies such as oral medications or physiotherapy is being customized based on disease progression
- Advanced diagnostic tools are being integrated into clinical workflows to assess blink rates, eyelid spasms, and facial muscle activity, enabling early diagnosis and timely intervention. These technologies support improved monitoring of treatment efficacy and help in optimizing repeat injection schedules
- The shift toward patient-centric treatment planning is encouraging pharmaceutical companies and medical device manufacturers to develop more specialized solutions for managing blepharospasm. This includes long-acting botulinum toxin formulations and wearable devices for real-time symptom monitoring
- This trend toward targeted and personalized care is fundamentally reshaping treatment protocols in the blepharospasm market. As healthcare systems increasingly emphasize outcomes-based approaches, providers and manufacturers are aligning to deliver highly individualized therapeutic solutions
- The demand for personalized and precision-based treatment options is growing rapidly across neurology and ophthalmology clinics worldwide, as physicians and patients alike seek improved quality of life and long-term symptom control
Blepharospasm Treatment Market Dynamics
Driver
“Growing Need Due to Rising Diagnosis Rates and Advancements in Neurological Treatment”
- The increasing awareness and diagnosis of neurological disorders, particularly dystonias such as blepharospasm, coupled with advancements in botulinum toxin formulations and oral therapies, is significantly driving the demand for effective blepharospasm treatment
- For instance, in April 2024, Revance Therapeutics announced expanded distribution of its FDA-approved botulinum toxin therapy, Daxxify, for therapeutic indications including blepharospasm, enhancing treatment accessibility across neurology clinics. Such initiatives by key players are expected to drive the Blepharospasm Treatment industry growth in the forecast period
- As patients and healthcare providers become more aware of the burden of chronic, involuntary eyelid spasms, there is a growing demand for safe and long-acting treatment solutions that improve quality of life and daily functioning
- Furthermore, the increasing geriatric population and rising incidence of drug-induced and idiopathic dystonias are contributing to the expansion of the patient pool, encouraging further R&D and product innovations
- The convenience and effectiveness of botulinum toxin injections, along with expanding clinical adoption of minimally invasive approaches and emerging oral drug formulations, are key factors propelling the growth of the blepharospasm treatment market in both hospital and outpatient settings. The rise of telemedicine and specialty neuro-ophthalmology services is also improving patient access to timely diagnosis and care
Restraint/Challenge
“High Treatment Costs and Limited Long-Term Therapies”
- The high cost of repeated botulinum toxin injections and limited access to long-term treatment strategies remain significant challenges to broader market adoption. The recurring nature of therapy—often every 3–4 months—can pose a financial burden on patients without sufficient insurance coverage
- For instance, delays in reimbursement approvals and geographic disparities in access to specialist care have made it difficult for patients in rural or underserved areas to receive consistent treatment.
- Addressing these cost and access issues through expanded insurance coverage, value-based pricing, and public health programs will be critical for sustaining treatment adherence and improving patient outcomes
- In addition, the lack of curative therapies and a narrow pipeline of novel pharmacological treatments highlights the need for continued innovation. Most current options focus on symptom control rather than disease modification, limiting long-term efficacy for some patient groups
- While ongoing research into gene therapy and new neuromodulators offers future potential, current reliance on symptomatic management and the relatively high procedural costs still present barriers to wider adoption, especially in emerging markets
- Overcoming these challenges will require a multipronged approach involving payer engagement, investment in R&D, patient assistance programs, and improved awareness among primary care providers and ophthalmologists regarding early diagnosis and referral to specialists
Blepharospasm Treatment Market Scope
The blepharospasm treatment market is segmented on the basis of type, treatment type, and distribution channel.
• By Type
On the basis of type, the blepharospasm treatment market is segmented into primary blepharospasm and secondary blepharospasm. The primary blepharospasm segment held the largest market revenue share of 62.3% in 2024, owing to its higher prevalence as an idiopathic condition with no underlying disease. Increasing awareness, better clinical diagnosis, and more patients seeking treatment contribute to the segment’s dominance.
The secondary blepharospasm segment is expected to witness the fastest CAGR of 8.4% from 2025 to 2032, driven by growing cases related to drug side effects, neurological disorders, and trauma-related facial dystonias.
• By Treatment Type
On the basis of treatment type, the blepharospasm treatment market is segmented into injections and oral medications. The injections segment dominated the market with the largest revenue share of 78.5% in 2024, primarily due to the widespread use of botulinum toxin type A injections as the gold standard treatment for blepharospasm. The therapy's proven efficacy, minimal invasiveness, and long-lasting effects support its strong adoption across clinical settings.
The oral medications segment is anticipated to grow at a fastest CAGR of 7.1% during the forecast period, driven by ongoing R&D in neuromodulators and anticholinergic agents offering adjunctive or alternative relief for patients unresponsive to injections.
• By Distribution Channel
On the basis of distribution channel, the blepharospasm treatment market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment captured the largest market revenue share of 54.6% in 2024, attributed to the fact that botulinum toxin injections are typically administered in specialized clinics or hospitals, with medications dispensed directly through in-house pharmacies.
The Online Pharmacy segment is projected to grow at the fastest CAGR of 9.3% from 2025 to 2032, driven by increasing patient preference for convenience, doorstep delivery, and availability of follow-up oral medications and supportive care products online.
Blepharospasm Treatment Market Regional Analysis
- North America dominated the blepharospasm treatment market with the largest revenue share of 41.8% in 2024, attributed to early adoption of botulinum toxin therapies, availability of advanced healthcare infrastructure, and a strong presence of leading pharmaceutical and device manufacturers
- U.S. continues to lead regional growth due to increased diagnosis rates, high awareness among patients, and favorable reimbursement policies
- The region’s leadership is also supported by the presence of key pharmaceutical players and favorable reimbursement policies for botulinum toxin treatments
U.S. Blepharospasm Treatment Market Insight
The U.S. blepharospasm treatment market captured the largest revenue share of 76% within North America in 2024, fueled by high patient awareness, early diagnosis, and the wide availability of FDA-approved treatments such as botulinum toxin. Increasing adoption of minimally invasive procedures and a strong neurologist network contribute to robust market growth. In addition, ongoing clinical trials and research initiatives further strengthen the U.S. position in the global market.
Europe Blepharospasm Treatment Market Insight
The Europe blepharospasm treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising demand for non-surgical therapeutic options and the growing aging population prone to neurological disorders. Increasing healthcare expenditure, especially in countries such as Germany, France, and the U.K., is accelerating access to effective treatment solutions.
U.K. Blepharospasm Treatment Market Insight
The U.K. blepharospasm treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the expanding use of botulinum toxin in both NHS and private healthcare facilities. A greater focus on movement disorder clinics and increasing public awareness campaigns about dystonia-related conditions are stimulating demand for timely diagnosis and treatment.
Germany Blepharospasm Treatment Market Insight
The Germany blepharospasm treatment market is expected to expand at a considerable CAGR during the forecast period, attributed to advancements in neurotherapeutics and the availability of high-quality healthcare infrastructure. Germany’s strong commitment to medical research and innovation is fostering the development of novel treatment regimens for blepharospasm.
Asia-Pacific Blepharospasm Treatment Market Insight
The Asia-Pacific blepharospasm treatment market is poised to grow at the fastest CAGR of 8.5% from 2025 to 2032, driven by rising neurological disease prevalence, expanding healthcare access, and increased investment in hospital infrastructure. Countries such as China, Japan, and India are seeing a surge in patient education, improving early diagnosis and expanding the adoption of re-injection therapies such as botulinum toxin.
Japan Blepharospasm Treatment Market Insight
The Japan blepharospasm treatment market is witnessing steady growth, driven by the country's advanced healthcare system and cultural openness to innovative medical treatments. With a rapidly aging population and a strong preference for precision medicine, Japan is seeing a rising number of patients receiving long-term management for blepharospasm through outpatient neurology clinics.
China Blepharospasm Treatment Market Insight
The China blepharospasm treatment market accounted for the largest revenue share within Asia-Pacific in 2024, fueled by a rising middle-class population, increased healthcare spending, and strong local pharmaceutical manufacturing. Government support for neurological care and expanding insurance coverage is enabling greater access to approved treatment protocols for movement disorders.
Blepharospasm Treatment Market Share
The blepharospasm treatment industry is primarily led by well-established companies, including:
- Ipsen Pharma (France)
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Abbott (U.S.)
- Eisai Co., Ltd. (Japan)
- Advanz Pharmaceutical Investors Inc. (U.K.)
- Sanofi (U.S.)
- Unichem Laboratories (India)
- Unimark Healthcare Ltd. (India)
- Axis Life Science Pvt. Ltd. (India)
- Zydus Group (India)
- Merz Pharma (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- IPCA Laboratories Ltd. (India)
- Wockhardt Limited (India)
Latest Developments in Global Blepharospasm Treatment Market
- In July 2024, Merz Pharmaceuticals received FDA approval for an expanded indication of Xeomin (incobotulinumtoxinA) to include the treatment of blepharospasm alongside existing indications such as cervical dystonia and sialorrhea. This regulatory milestone reinforces Merz’s leadership in the botulinum toxin market and enhances access to a proven therapeutic option for patients suffering from involuntary eyelid spasms
- In February 2025, a clinical study published in ScienceDirect introduced a new combined preseptal and pretarsal botulinum toxin injection protocol for treating blepharospasm. The findings showed significant improvements in symptom relief and patient satisfaction compared to traditional injection techniques, representing a meaningful advancement in clinical practice
- In 2024, the Centers for Medicare & Medicaid Services (CMS) issued updated guidelines for the clinical use and reimbursement of botulinum toxin treatments for blepharospasm. These guidelines emphasized the use of standardized assessment tools such as the Jankovic Rating Scale (JRS) and Blepharospasm Disability Index (BSDI), while also setting clear parameters on dosing and documentation to ensure safe and effective use
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.