Global Budd Chiari Syndrome Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.74 Billion
USD
2.29 Billion
2024
2032
USD
1.74 Billion
USD
2.29 Billion
2024
2032
| 2025 –2032 | |
| USD 1.74 Billion | |
| USD 2.29 Billion | |
|
|
|
|
Budd - Chiari Syndrome Treatment Market Size
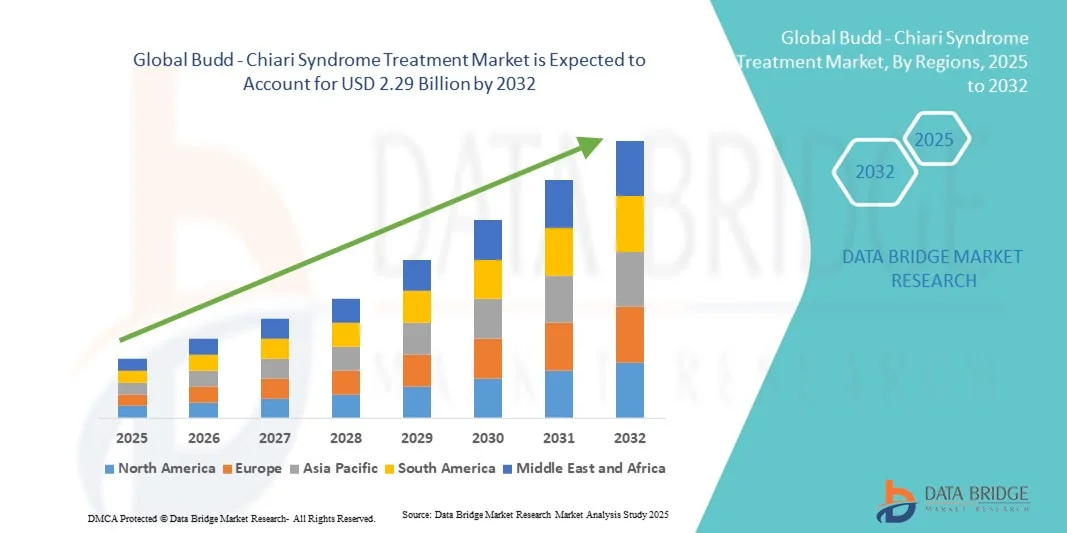
- The global budd - chiari syndrome treatment market size was valued at USD 1.74 billion in 2024 and is expected to reach USD 2.29 billion by 2032, at a CAGR of 3.50% during the forecast period
- The market growth is largely fueled by the increasing prevalence of liver-related disorders and advancements in diagnostic and therapeutic technologies, which have enhanced early detection and treatment outcomes for budd-chiari syndrome
- Furthermore, growing awareness among healthcare professionals and patients, coupled with the rising availability of novel drug formulations and minimally invasive surgical procedures, is establishing budd-chiari syndrome treatment as a crucial focus area in hepatology. These converging factors are accelerating the adoption of budd-chiari syndrome treatment solutions, thereby significantly boosting the industry's growth
Budd - Chiari Syndrome Treatment Market Analysis
- Budd-Chiari Syndrome Treatment, encompassing pharmacological, surgical, and interventional therapies, has become an essential component of modern hepatology care in both developed and emerging healthcare systems due to its effectiveness in managing hepatic venous outflow obstruction and improving patient outcomes.
- The growing demand for budd-chiari syndrome treatment is primarily fueled by the increasing prevalence of liver disorders, advancements in imaging and diagnostic techniques, and a rising emphasis on early disease management and precision medicine
- North America dominated the budd-chiari syndrome treatment market with the largest revenue share of 38.7% in 2024, supported by well-established healthcare infrastructure, favorable reimbursement policies, and strong research and development activities, particularly in the U.S., where increased clinical trials and awareness programs are driving treatment adoption
- Asia-Pacific is expected to be the fastest-growing region in the budd-chiari syndrome treatment market during the forecast period due to the rising incidence of liver diseases, improving healthcare access, and growing investment in specialized treatment centers across countries such as China and India
- The laboratory tests segment dominated the largest market revenue share of 63.4% in 2024, driven by their critical role in confirming diagnoses through imaging, liver function assessments, and hematologic evaluations
Report Scope and Budd - Chiari Syndrome Treatment Market Segmentation
|
Attributes |
Budd - Chiari Syndrome Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Budd - Chiari Syndrome Treatment Market Trends
Advancements in Precision Medicine and Interventional Therapies
- A significant and accelerating trend in the global budd–chiari syndrome treatment market is the integration of precision medicine, advanced diagnostics, and minimally invasive interventional procedures to enhance patient outcomes. This development is redefining treatment standards for rare hepatic vascular disorders and is driving innovation among healthcare providers and medical device manufacturers
- For instance, in March 2023, researchers at the University of Tokyo introduced a computational hemodynamic modeling system that helps predict the efficacy of shunt or stent placement in Budd–Chiari patients before surgery, supporting more accurate and individualized treatment decisions. Similarly, advanced imaging modalities such as contrast-enhanced MRI and Doppler ultrasonography are being increasingly used to provide detailed visualization of hepatic vein obstruction, improving diagnostic precision and treatment planning
- The increasing adoption of transjugular intrahepatic portosystemic shunt (TIPS) and endovascular stenting techniques is also transforming therapeutic approaches by reducing the need for invasive surgeries and improving long-term survival rates. For instance, in August 2024, a multicenter European study reported improved outcomes with novel covered stents specifically designed for hepatic outflow obstruction, marking a major advancement in interventional radiology for Budd–Chiari management
- Furthermore, the growing incorporation of AI-assisted diagnostic tools and biomarker-based risk stratification methods is allowing clinicians to better identify prothrombotic conditions associated with the disease, supporting earlier and more personalized intervention. This trend toward individualized, data-driven care is fundamentally reshaping both the clinical and commercial landscape of Budd–Chiari Syndrome treatment worldwide
- The ongoing development of liver support devices, next-generation stents, and bioengineered vascular grafts reflects a broader movement toward intelligent, patient-specific therapies aimed at minimizing complications and improving recovery times for affected individuals
Budd - Chiari Syndrome Treatment Market Dynamics
Driver
Rising Detection Rates and Expanding Access to Advanced Therapies
- The increasing recognition and diagnosis of budd–chiari syndrome, supported by technological advancements and expanding clinical awareness, is a major driver of market growth. With the condition being historically underdiagnosed, the availability of improved imaging and genetic testing tools has significantly increased detection rates across developed and emerging economies
- For instance, in April 2024, GE HealthCare launched an upgraded liver imaging suite with enhanced vascular assessment capabilities designed for early detection of hepatic venous outflow obstruction, aiding clinicians in diagnosing Budd–Chiari Syndrome more efficiently. Such innovations are improving clinical confidence and expanding treatment uptake
- Furthermore, advances in interventional radiology, anticoagulant therapy, and liver transplantation techniques have strengthened the treatment landscape. For instance, in February 2023, the Cleveland Clinic announced the success of a new minimally invasive shunt technique designed to reduce complications in Budd–Chiari patients with secondary liver cirrhosis, highlighting the growing role of specialized centers in driving better outcomes
- Continuous investments by healthcare systems, academic institutions, and pharmaceutical companies in rare disease programs are accelerating clinical research and development of targeted therapies. For instance, in October 2022, the U.K. Rare Disease Research Consortium initiated a grant to study genetic markers linked to Budd–Chiari predisposition, aiming to support precision-based drug development
- The growing number of interventional and pharmacological options, combined with increased healthcare access in Asia-Pacific and the Middle East regions, is further supporting the expansion of this market as patients seek safer, more effective alternatives to conventional surgical procedures
Restraint/Challenge
High Treatment Costs and Limited Clinical Expertise
- Despite significant progress, the budd–chiari syndrome treatment market faces notable challenges stemming from high treatment costs, limited clinical expertise, and the rarity of the condition. The complex nature of the disease demands specialized interventional radiology and hepatology skills, which are often concentrated in major tertiary centers, limiting accessibility for patients in low-resource regions
- For instance, in December 2023, a study published by the European Association for the Study of the Liver (EASL) highlighted that nearly 40% of patients in developing regions experience delays in diagnosis due to limited imaging access and lack of specialized expertise, resulting in poorer clinical outcomes
- In addition, the high costs associated with advanced imaging, endovascular interventions, and liver transplantation create affordability barriers for both patients and healthcare providers. For instance, covered stent procedures for Budd–Chiari can cost several times more than traditional surgical decompression methods, making them less accessible in middle- and low-income countries
- Another significant challenge is the absence of standardized treatment guidelines, given the rarity and heterogeneity of the syndrome. This results in variations in therapeutic approaches, making clinical decision-making inconsistent across regions. For instance, while the U.S. and parts of Europe emphasize early TIPS intervention, many Asian centers still rely on conservative medical management due to cost and expertise limitations
- Furthermore, the scarcity of large-scale clinical trials due to the small patient population limits robust evidence generation and slows the regulatory approval of novel therapies. Overcoming these challenges through international collaboration, cost-effective interventional innovations, and clinician training initiatives will be crucial for ensuring sustainable market growth in the years ahead
Budd - Chiari Syndrome Treatment Market Scope
The market is segmented on the basis of diagnosis, treatment, gender, age group, and end user.
- By Diagnosis
On the basis of diagnosis, the Budd–Chiari Syndrome Treatment market is segmented into physical examinations and laboratory tests. The laboratory tests segment dominated the largest market revenue share of 63.4% in 2024, driven by their critical role in confirming diagnoses through imaging, liver function assessments, and hematologic evaluations. Techniques such as Doppler ultrasound, MRI, and CT scans are widely used to detect hepatic vein obstruction with high precision, making laboratory testing the preferred diagnostic approach. The growing adoption of advanced imaging modalities, technological advancements in diagnostic tools, and the increasing emphasis on early and accurate detection of liver-related disorders further enhance the dominance of this segment. In addition, laboratory tests enable clinicians to monitor disease progression and evaluate treatment effectiveness, ensuring personalized patient management.
The physical examinations segment is anticipated to witness the fastest CAGR of 10.9% from 2025 to 2032, driven by the increasing emphasis on primary screening and early detection in healthcare facilities. Physical assessments remain vital for identifying initial symptoms such as ascites, hepatomegaly, and abdominal discomfort, prompting further diagnostic confirmation through imaging. With rising awareness among healthcare professionals about the importance of comprehensive physical evaluations in liver disease management, the segment is gaining prominence. Integration of point-of-care diagnostics with clinical evaluation and enhanced accessibility in low-resource settings also contribute to this segment’s accelerated growth.
- By Treatment
On the basis of treatment, the Budd–Chiari Syndrome Treatment market is segmented into therapy, medication, and surgery. The medication segment dominated the largest market revenue share of 49.6% in 2024, primarily driven by the widespread use of anticoagulants, thrombolytics, and diuretics to manage symptoms and prevent disease progression. Pharmacological management remains the first-line approach for most patients, emphasizing non-invasive control of thrombosis and fluid accumulation. Increasing availability of novel therapeutic drugs, improved patient adherence, and ongoing clinical research into targeted molecules enhance segment growth. Moreover, the rising global prevalence of chronic liver diseases and an expanding patient population requiring long-term medical management further reinforce the dominance of medication-based treatments.
The surgery segment is projected to witness the fastest CAGR of 12.8% from 2025 to 2032, owing to the growing success of surgical and interventional procedures such as transjugular intrahepatic portosystemic shunt (TIPS), angioplasty, and liver transplantation. Advancements in minimally invasive techniques and improved postoperative outcomes are driving adoption among severe or refractory cases. The rising availability of specialized hepatology centers and the increasing expertise of surgeons in managing complex hepatic venous obstructions also support this segment’s rapid growth. In addition, growing health insurance coverage for high-cost procedures further encourages patients to opt for surgical interventions.
- By Gender
On the basis of gender, the Budd–Chiari Syndrome Treatment market is segmented into male and female. The female segment dominated the largest market revenue share of 56.1% in 2024, largely due to the higher prevalence of Budd–Chiari Syndrome among women, particularly those using oral contraceptives or undergoing hormonal therapy. Hormonal influences on coagulation and increased risk during pregnancy also contribute to higher incidence rates in females. Growing awareness regarding women’s liver health, improved access to diagnostic facilities, and gender-specific research on hepatic disorders further support this segment’s leading position. Moreover, targeted treatment approaches and better screening protocols for female patients strengthen its market share.
The male segment is expected to witness the fastest CAGR of 9.7% from 2025 to 2032, driven by rising cases linked to underlying conditions such as myeloproliferative disorders and lifestyle-related risk factors. Increased participation of men in screening programs and greater awareness of liver health are fostering early diagnosis and treatment adoption. Furthermore, the development of gender-neutral treatment protocols and clinical trials focusing on male patients are enhancing therapeutic effectiveness, thereby fueling segment growth. In addition, advancements in personalized medicine and targeted therapies are improving treatment outcomes specifically for male patients. Growing collaboration between healthcare providers and patient advocacy groups is also supporting awareness and early intervention efforts in this segment.
- By Age Group
On the basis of age group, the Budd–Chiari Syndrome Treatment market is segmented into 0–20 years, 20–40 years, 40–60 years, and above 60 years. The 20–40 years segment dominated the largest market revenue share of 41.8% in 2024, as this age group represents a significant proportion of patients affected by hypercoagulable states and hormonal changes. The high prevalence of contraceptive use, pregnancy-related risks, and metabolic disorders in this age group contribute to its dominance. In addition, increasing awareness, accessibility to diagnostic technologies, and early initiation of therapy support the segment’s strong performance. Healthcare professionals’ focus on preventive care and the growing number of specialized liver clinics further enhance this demographic’s contribution to market revenue.
The above 60 years segment is projected to witness the fastest CAGR of 11.6% from 2025 to 2032, attributed to the growing geriatric population worldwide and their heightened susceptibility to chronic liver diseases and vascular complications. The increasing burden of comorbidities, polypharmacy, and age-related venous insufficiencies has amplified diagnostic and treatment needs in this group. Enhanced access to geriatric care facilities, advancements in minimally invasive surgical options, and growing adoption of supportive therapies contribute to this segment’s rapid expansion. Moreover, increasing government focus on elderly healthcare and liver disease management programs further bolsters the segment’s growth prospects.
- By End User
On the basis of end user, the Budd–Chiari Syndrome Treatment market is segmented into hospitals, specialty clinics, and others. The hospitals segment accounted for the largest market revenue share of 58.9% in 2024, driven by the availability of advanced diagnostic equipment, multidisciplinary care teams, and comprehensive treatment infrastructure. Hospitals serve as primary centers for managing moderate to severe cases requiring surgical or interventional procedures, ensuring high patient throughput. Increasing hospital admissions for liver-related complications, coupled with improved reimbursement policies and government initiatives for healthcare modernization, further strengthen this segment’s dominance.
The specialty clinics segment is expected to witness the fastest CAGR of 13.2% from 2025 to 2032, propelled by the rising preference for personalized and specialized care. These clinics offer focused hepatology services, faster diagnosis, and individualized treatment plans, enhancing patient satisfaction and clinical outcomes. Growing private sector investments, technological integration for remote monitoring, and the expansion of outpatient services in emerging economies are accelerating this segment’s growth. In addition, the convenience of shorter waiting times and cost-effective procedures further supports their increasing adoption.
Budd - Chiari Syndrome Treatment Market Regional Analysis
- North America dominated the budd–chiari syndrome treatment market with the largest revenue share of 38.7% in 2024, supported by a well-established healthcare infrastructure, favorable reimbursement policies, and strong research and development activities
- The market captured the majority of the regional market share, fueled by increasing clinical trials, awareness programs, and rising adoption of advanced diagnostic and treatment modalities. High patient awareness, government-led health initiatives, and the presence of leading hospitals and specialty clinics contribute to the market’s robust growth
- Moreover, investments in novel therapies, interventional procedures, and multidisciplinary treatment approaches further consolidate North America’s leadership in the global market
U.S. Budd–Chiari Syndrome Treatment Market Insight
The U.S. budd–chiari syndrome treatment market captured the largest revenue share in 2024 within North America, driven by increasing clinical trials, awareness campaigns, and a rising preference for early diagnosis and advanced treatment options. The country’s strong healthcare infrastructure, supportive reimbursement policies, and high R&D investments in hepatology are significantly contributing to market growth. The growing number of specialized hospitals and liver treatment centers, alongside government-led initiatives for liver disease awareness, further propels the adoption of Budd–Chiari Syndrome treatments.
Europe Budd–Chiari Syndrome Treatment Market Insight
The Europe budd–chiari syndrome treatment market is projected to expand at a substantial CAGR throughout the forecast period, driven by increasing awareness of liver diseases, stringent healthcare regulations, and rising adoption of advanced treatment modalities. The increase in specialized healthcare facilities, coupled with the demand for early diagnosis and multidisciplinary care, is fostering market growth. European consumers are increasingly seeking high-quality medical care for liver-related disorders, enhancing the adoption of innovative therapies and interventions.
U.K. Budd–Chiari Syndrome Treatment Market Insight
The U.K. budd–chiari syndrome treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing awareness of liver diseases, rising demand for specialized treatment centers, and early diagnosis initiatives. Growing patient education programs and government health campaigns are supporting the uptake of advanced treatment options.
Germany Budd–Chiari Syndrome Treatment Market Insight
The Germany Budd–Chiari Syndrome Treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by well-developed healthcare infrastructure, increasing awareness of liver disorders, and the availability of specialized hospitals and clinics. Rising adoption of advanced diagnostic technologies and minimally invasive treatment options further supports market growth.
Asia-Pacific Budd–Chiari Syndrome Treatment Market Insight
The Asia-Pacific budd–chiari syndrome treatment market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by the rising incidence of liver diseases, improving healthcare access, and growing investment in specialized treatment centers across countries such as China and India. Increasing healthcare expenditure, expanding hospital networks, and government initiatives promoting liver disease management are key factors propelling market growth in the region.
Japan Budd–Chiari Syndrome Treatment Market Insight
The Japan budd–chiari syndrome treatment market is gaining momentum due to the country’s high awareness of liver health, an aging population, and growing demand for advanced treatment options. Increasing adoption of minimally invasive procedures and the presence of specialized hepatology centers are contributing to market growth.
China Budd–Chiari Syndrome Treatment Market Insight
The China budd–chiari syndrome treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the rising prevalence of liver disorders, rapid urbanization, and increasing healthcare infrastructure. Growing government focus on liver disease management, expansion of specialized hospitals, and rising patient awareness are driving adoption of advanced treatment options.
Budd - Chiari Syndrome Treatment Market Share
The Budd - Chiari Syndrome Treatment industry is primarily led by well-established companies, including:
- Sequana Medical NV (Belgium)
- PharmaCyte Biotech Inc. (U.S.)
- BioVie Inc. (U.S.)
- Medtronic (Ireland)
- Eurolyser Diagnostica GmbH (Germany)
- GENERi BIOTECH (Germany)
- Abbott (U.S.)
- TrimGen Corporation (U.S.
- ACON Laboratories, Inc. (U.S.)
- CoaguSense, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Dr. Reddy’s Laboratories Ltd. (India)
- B. Braun Melsungen AG (Germany)
- Pfizer Inc. (U.S.)
- Fresenius SE & Co. KGaA (Germany)
- Bioberica S.A.U. (Spain)
- Baxter (U.S.)
- OPOCRIN S.P.A. (Italy)
- Aspen Holdings (South Africa)
Latest Developments in Global Budd - Chiari Syndrome Treatment Market
- In April 2023, a comprehensive review published in Diagnostics provided an updated overview of the diagnosis and management of Budd–Chiari Syndrome, highlighting advancements in imaging techniques and interventional therapies. The review emphasized the role of contrast-enhanced MRI and Doppler ultrasonography in improving diagnostic accuracy and the increasing utilization of transjugular intrahepatic portosystemic shunt (TIPS) procedures for treatment
- In September 2024, a study published in Diagnostics discussed the state-of-the-art imaging methods for diagnosing Budd–Chiari Syndrome, focusing on the advancements in imaging modalities that enhance the detection and characterization of hepatic venous outflow obstruction
- In May 2025, the U.S. Food and Drug Administration approved belzutifan (Welireg) for adults and adolescents aged 12 and older with locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma, marking the first oral treatment authorized for these tumors. While not directly related to Budd–Chiari Syndrome, this approval reflects ongoing advancements in the treatment of rare vascular and hematologic disorders
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.