Global Canavan Disease Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
556.71 Million
USD
756.05 Million
2024
2032
USD
556.71 Million
USD
756.05 Million
2024
2032
| 2025 –2032 | |
| USD 556.71 Million | |
| USD 756.05 Million | |
|
|
|
|
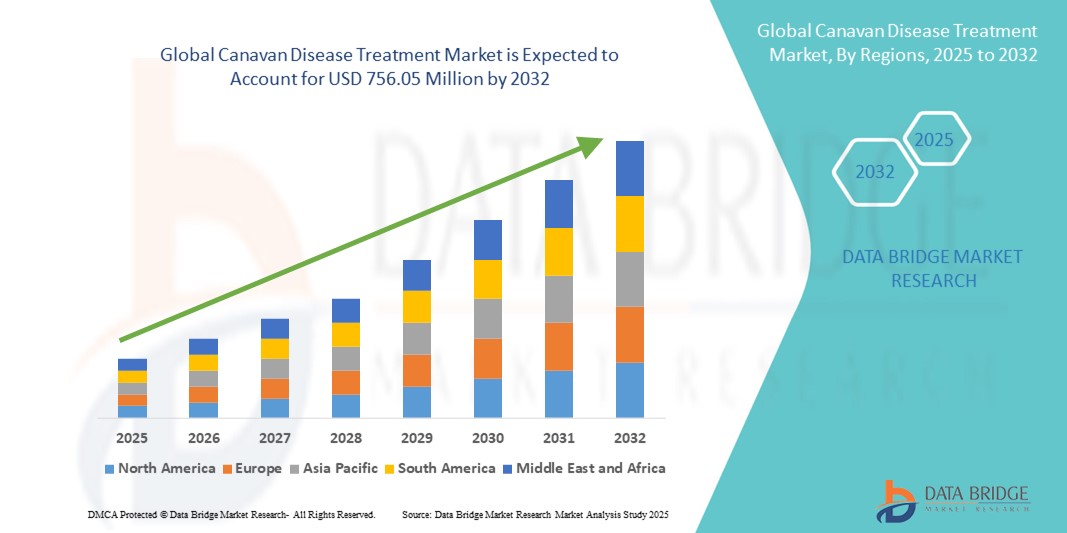
Canavan Disease Treatment Market Size
- The global Canavan disease treatment market size was valued at USD 556.71 million in 2024 and is expected to reach USD 756.05 million by 2032, at a CAGR of 3.9% during the forecast period
- The market growth is largely fueled by rising research into gene therapies, enzyme replacement solutions, and supportive treatments targeting rare neurodegenerative disorders, particularly those affecting pediatric populations
- Furthermore, increasing investment in orphan drug development, supportive regulatory frameworks, and early diagnostic advancements are driving significant innovation in the Canavan disease treatment landscape. These factors are collectively fostering stronger treatment pipelines, thereby accelerating the market’s overall expansion
Canavan Disease Treatment Market Analysis
- Canavan disease treatments, including gene therapy, enzyme replacement, and supportive care, are becoming increasingly essential in managing this rare and progressive neurological disorder, particularly in infants, as advancements aim to slow or halt disease progression by targeting underlying genetic mutations
- The growing demand for Canavan disease treatment is primarily fueled by increased rare disease awareness, rising investment in gene therapy research, and supportive regulatory frameworks that incentivize orphan drug development and accelerate clinical advancements
- North America dominated the Canavan disease treatment market with the largest revenue share of 40.5% in 2024, driven by its advanced research infrastructure, favorable reimbursement environment, and the presence of leading biotechnology firms and academic institutions actively engaged in clinical trials
- Asia-Pacific is expected to be the fastest growing region in the Canavan disease treatment market during the forecast period due to improving healthcare infrastructure, growing investment in rare disease diagnostics, and increasing awareness and diagnosis rates across emerging economies
- Gene therapy segment dominated the Canavan disease treatment market with a market share of 46.8% in 2024, driven by its long-term potential to correct the ASPA gene mutation and provide targeted therapeutic outcomes unmatched by traditional treatments
Report Scope and Canavan Disease Treatment Market Segmentation
|
Attributes |
Canavan Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Canavan Disease Treatment Market Trends
Advancements in Gene Therapy and Precision Medicine
- A significant and rapidly evolving trend in the global Canavan disease treatment market is the advancement of gene therapy approaches aimed at correcting the underlying ASPA gene mutation responsible for the disease. These innovative therapies offer hope for long-term disease modification and potentially curative outcomes, reshaping the treatment paradigm for rare neurodegenerative disorders
- For instance, the clinical development of investigational gene therapies such as ASPAration by Aspa Therapeutics is a major milestone, using adeno-associated viral vectors to deliver functional ASPA genes directly to the brain. Early-phase trials have shown promise in improving motor function and slowing disease progression in pediatric patients
- The broader application of precision medicine and next-generation sequencing (NGS) is also enabling earlier diagnosis and personalized treatment plans. Genetic testing has become more accessible, allowing clinicians to confirm Canavan disease at earlier stages, which is critical for effective intervention
- Collaborative efforts between biotech firms, academic research institutions, and regulatory agencies are accelerating the development of novel therapeutics. For instance, partnerships between companies such as Passage Bio and leading universities are facilitating gene therapy innovation for leukodystrophies including Canavan disease
- This shift toward disease-modifying and patient-specific therapies is fundamentally transforming expectations within the rare disease treatment landscape. As a result, regulatory bodies are offering expedited pathways such as Fast Track and Orphan Drug designation, encouraging further investment and innovation in Canavan disease therapies
- The demand for targeted, durable, and potentially curative treatments is growing across global markets, especially in regions with increasing awareness and access to advanced genetic diagnostics and therapeutic platforms
Canavan Disease Treatment Market Dynamics
Driver
Rising Research Investment and Orphan Drug Incentives
- The increasing global focus on rare diseases, coupled with expanding research investment in gene and enzyme replacement therapies, is a primary driver of growth in the Canavan disease treatment market
- For instance, in 2024, multiple companies, including Aspa Therapeutics and Passage Bio, received funding to advance clinical-stage gene therapies targeting Canavan disease, marking a major step forward in therapeutic development
- Supportive policy environments such as the U.S. Orphan Drug Act and Europe’s Orphan Medicinal Products Regulation offer benefits such as market exclusivity, tax credits, and streamlined approval processes, which are encouraging biotechnology companies to enter the rare disease space
- Growing awareness campaigns by patient advocacy groups and increasing diagnostic capabilities are improving early detection and treatment access, making Canavan disease a more actionable clinical target. The ability to intervene early with gene-based therapies is proving vital in delaying neurodegeneration and enhancing patient outcomes
- As pediatric neurologists and rare disease specialists increasingly adopt personalized approaches, the market is benefiting from a confluence of technological innovation, clinical need, and regulatory alignment
Restraint/Challenge
Clinical Trial Complexity and Cost of Treatment
- The development of Canavan disease therapies especially gene therapies faces challenges due to the complexity of conducting clinical trials in a small, pediatric patient population with limited natural history data
- For instance, achieving statistically significant outcomes requires global trial coordination and long-term follow-up, which increases costs and delays development timelines. In addition, ethical considerations for enrolling infants in early-phase trials further complicate recruitment
- The high cost of gene therapy development and administration remains a barrier, with some treatments projected to cost several hundred thousand dollars per patient, raising concerns about reimbursement and equitable access, particularly in developing regions
- Moreover, the manufacturing of viral vectors and maintaining treatment quality and safety at scale present logistical hurdles. Ensuring long-term efficacy and monitoring potential side effects also require sustained investment post-approval
- Overcoming these challenges will depend on continued cross-sector collaboration, public and private funding support, and the development of scalable, cost-effective platforms that ensure both clinical and commercial sustainability
Canavan Disease Treatment Market Scope
The market is segmented on the basis of treatment and end user.
- By Treatment
On the basis of treatment, the Canavan disease treatment market is segmented into gene therapy, drug therapy, and others. The gene therapy segment dominated the market with the largest revenue share of 46.8% in 2024, driven by its potential to provide long-lasting or curative effects by directly addressing the genetic mutation (ASPA gene) responsible for Canavan disease. Advancements in AAV (adeno-associated virus) vector technologies, increasing clinical trial activity, and favorable regulatory support such as orphan drug designations have further bolstered gene therapy’s position as the leading treatment modality. The ability of gene therapy to offer early intervention and delay neurodegeneration has made it a primary focus in both research and clinical settings.
The drug therapy segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by ongoing development of novel small-molecule treatments and supportive medications that can alleviate symptoms or slow disease progression. Increasing efforts to repurpose existing neurology drugs for Canavan disease, along with expanded access to enzyme replacement therapies and neuroprotective agents, are expected to contribute to significant growth in this segment. In addition, the lower cost and non-invasive nature of drug-based treatments may drive broader accessibility in regions with limited access to advanced gene therapies.
- By End User
On the basis of end user, the Canavan disease treatment market is segmented into hospitals, specialty clinics, ambulatory surgical centers, and others. The hospital segment held the largest revenue share in 2024, attributed to the availability of advanced diagnostic tools, multidisciplinary pediatric neurology teams, and specialized infrastructure for administering complex treatments such as gene therapy. Hospitals are also the primary sites for clinical trial participation, which remains crucial for Canavan disease due to the investigational nature of many therapies currently under development.
The specialty clinics segment is expected to experience the fastest CAGR from 2025 to 2032, driven by the growing demand for rare disease-focused care and the emergence of specialized genetic and metabolic clinics. These centers offer more personalized, long-term management for Canavan disease patients and often facilitate quicker diagnostic and treatment pathways. Increased collaboration between specialty clinics and biotech firms is further supporting patient access to emerging therapies, including those in early access or compassionate use programs.
Canavan Disease Treatment Market Regional Analysis
- North America dominated the Canavan disease treatment market with the largest revenue share of 40.5% in 2024, driven by its advanced research infrastructure, favorable reimbursement environment, and the presence of leading biotechnology firms and academic institutions actively engaged in clinical trials
- The region benefits from a well-established healthcare infrastructure, a high concentration of clinical trials, and robust funding from both public and private sectors dedicated to rare disease research and gene therapy innovation
- Widespread access to genetic testing, strong collaboration between biotech firms and academic institutions, and rising awareness of Canavan disease among healthcare providers and patient communities are collectively fostering early diagnosis and treatment adoption, solidifying North America's leadership in the global market
U.S. Canavan Disease Treatment Market Insight
The U.S. Canavan disease treatment market captured the largest revenue share of 81% in North America in 2024, fueled by robust R&D efforts in gene therapy and orphan drug development. The country’s advanced clinical infrastructure, presence of major biotech companies, and supportive policies under the Orphan Drug Act have accelerated therapeutic innovation. The growing number of rare disease-focused centers and access to early diagnostic tools such as genetic testing further enhance the nation’s leadership. Strong collaboration between academic institutions and biotech firms continues to drive clinical advancements in Canavan disease treatment.
Europe Canavan Disease Treatment Market Insight
The Europe Canavan disease treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing rare disease research funding and pan-European healthcare collaboration. The EU’s emphasis on orphan medicinal product regulations and cross-border research initiatives is fostering growth. Countries across the region are witnessing rising awareness of leukodystrophies, including Canavan disease, leading to earlier diagnosis and access to experimental therapies. The expansion of genetic screening programs and partnerships between research institutions and biotech firms is further supporting regional growth.
U.K. Canavan Disease Treatment Market Insight
The U.K. Canavan disease treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the nation’s proactive stance on rare disease management and expanded genomic initiatives under the NHS. With programs such as the Genomic Medicine Service enabling earlier diagnosis of conditions such as Canavan disease, the U.K. is paving the way for personalized treatment. Increasing clinical trial participation, public-private collaborations, and government-backed funding for orphan drug research are further propelling the market forward.
Germany Canavan Disease Treatment Market Insight
The Germany Canavan disease treatment market is expected to expand at a considerable CAGR during the forecast period, driven by strong investment in biotechnology, rare disease awareness campaigns, and a well-established healthcare infrastructure. Germany’s focus on innovation and participation in EU-wide rare disease programs make it a leading contributor to Canavan research. Increased collaboration between hospitals and research institutes, along with growing access to advanced gene therapy platforms, supports rising adoption of disease-modifying treatments.
Asia-Pacific Canavan Disease Treatment Market Insight
The Asia-Pacific Canavan disease treatment market is poised to grow at the fastest CAGR of 24% during the forecast period of 2025 to 2032, fueled by rapid improvements in diagnostic capabilities and healthcare access across key economies such as China, Japan, and India. Government initiatives to promote rare disease registries, along with increasing investment in genomic research, are supporting early detection and treatment. The expanding presence of international biotech firms and regional research partnerships is opening pathways for innovative therapies in the APAC region.
Japan Canavan Disease Treatment Market Insight
The Japan Canavan disease treatment market is gaining momentum due to the country’s emphasis on precision medicine and integration of rare diseases into national health strategies. Japan’s robust infrastructure for genetic research and early diagnosis plays a key role in driving adoption of advanced therapies. With rising support for pediatric neurology and the development of local clinical trial networks, the country is actively advancing patient access to emerging gene therapies and collaborative research programs in the leukodystrophy space.
India Canavan Disease Treatment Market Insight
The India Canavan disease treatment market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to a growing medical genetics sector, expanding middle class, and heightened focus on pediatric healthcare. India is witnessing increasing awareness around rare diseases, supported by initiatives such as the National Policy for Rare Diseases. The availability of affordable genetic testing, expanding research institutions, and a strong domestic pharmaceutical industry are positioning India as a key player in driving regional market growth for Canavan disease treatment.
Canavan Disease Treatment Market Share
The Canavan disease treatment industry is primarily led by well-established companies, including:
- Aspa Therapeutics, Inc. (U.S.)
- Passage Bio, Inc. (U.S.)
- REGENXBIO Inc. (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Taysha Gene Therapies, Inc. (U.S.)
- Orchard Therapeutics plc (U.K.)
- Abeona Therapeutics Inc. (U.S.)
- Denali Therapeutics Inc. (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- BridgeBio Pharma, Inc. (U.S.)
- Voyager Therapeutics, Inc. (U.S.)
- Avrobio, Inc. (U.S.)
- MeiraGTx Holdings plc (U.K.)
- Genethon (France)
- uniQure N.V. (Netherlands)
- Santhera Pharmaceuticals Holding AG (Switzerland)
- JCR Pharmaceuticals Co., Ltd. (Japan)
What are the Recent Developments in Global Canavan Disease Treatment Market?
- In July 2025, Myrtelle Inc., a clinical-stage gene therapy company, launched commercial-stage manufacturing of MYR‑101, an investigational rAAV gene therapy targeting oligodendrocytes for Canavan disease. Developed to restore ASPA enzyme function, MYR‑101 has received multiple regulatory designations, including Orphan Drug and Fast Track. This advancement emphasizes Myrtelle’s strategic commitment to addressing the underlying genetic cause of the disease and supporting large-scale global access through enhanced manufacturing capabilities
- In June 2025, BridgeBio Pharma Inc., through its affiliate Aspa Therapeutics, presented promising 12-month data from the high-dose cohort of its Phase 1/2 CANaspire trial for BBP‑812. The AAV9-based gene therapy demonstrated significant reductions in N‑acetylaspartate (NAA) levels and improvements in motor function, indicating strong therapeutic potential. These findings reinforce BridgeBio’s commitment to developing the first approved treatment for Canavan disease while setting the stage for future regulatory submissions
- In May 2025, Myrtelle announced positive interim results from its Phase 1/2 clinical trial evaluating rAAV‑Olig001‑ASPA in pediatric patients. The data revealed meaningful structural and functional improvements in brain white matter, motor skills, and myelination markers. These outcomes underline the therapeutic promise of targeted gene therapy and Myrtelle’s progress in advancing treatment options for rare leukodystrophies
- In January 2025, the CANaspire trial initiated broader enrollment across North America and Europe for infants under 30 months. The expansion aims to evaluate safety and efficacy across diverse populations, supporting the global development of BBP‑812. This step marks a significant milestone for BridgeBio and Aspa Therapeutics, reinforcing their global leadership in advancing precision therapies for ultra-rare neurological disorders
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.