Global Cancer Biologics Market
Market Size in USD Billion
CAGR :
% 
 USD
122.55 Billion
USD
195.62 Billion
2024
2032
USD
122.55 Billion
USD
195.62 Billion
2024
2032
| 2025 –2032 | |
| USD 122.55 Billion | |
| USD 195.62 Billion | |
|
|
|
Cancer Biologics Market Analysis
The cancer biologics market is rapidly evolving, driven by remarkable advancements in biotechnology and growing demand for targeted treatments. Biologic therapies, which harness the body's immune system to fight cancer, are gaining significant momentum due to their ability to deliver more effective, personalized treatments compared to traditional therapies. The surge in innovative therapies such as monoclonal antibodies, CAR T-cell therapies, and immune checkpoint inhibitors is reshaping the landscape, offering hope for previously hard-to-treat cancers.
With a shift toward precision medicine, there’s an increasing focus on tailoring treatments to individual genetic profiles, optimizing therapeutic outcomes, and minimizing side effects. This trend is bolstered by ongoing research, the growing understanding of cancer biology, and breakthroughs in genetic engineering and immunotherapy.
However, the market faces challenges such as high treatment costs, regulatory hurdles, and concerns regarding long-term efficacy and safety. Despite this, the ongoing investments by pharmaceutical companies, increasing partnerships with research institutions, and a wave of new product approvals suggest a promising future.
The cancer biologics market's growth is underscored by strong clinical trial pipelines and expanding healthcare infrastructure, offering tremendous potential for innovation. This dynamic space is evolving rapidly, with new breakthroughs continuing to redefine how cancer is treated and ultimately paving the way for improved patient outcomes.
Cancer Biologics Market Size
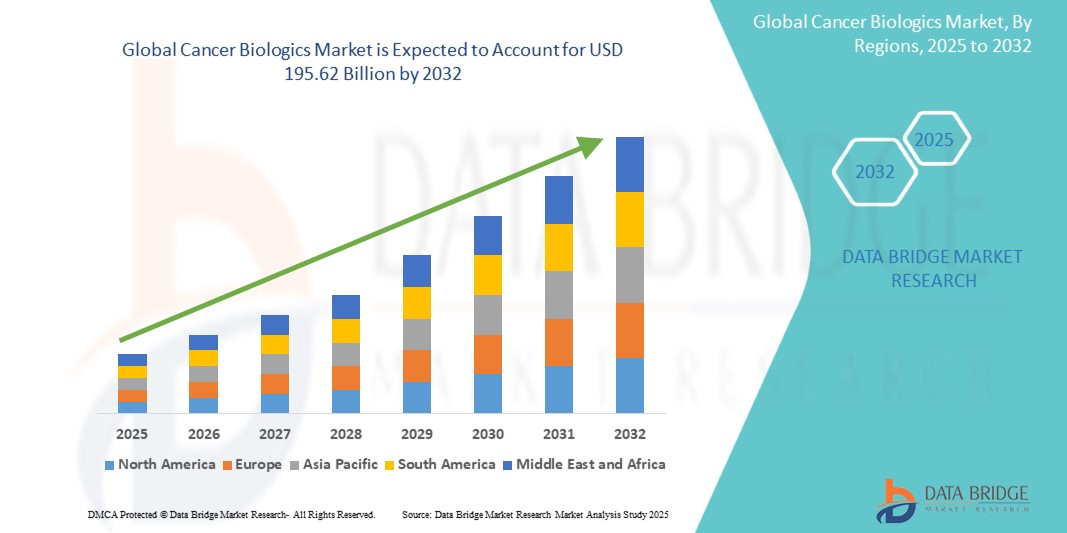
The global cancer biologics market size was valued at USD 122.55 billion in 2024 and is projected to reach USD 195.62 billion by 2032, with a CAGR of 6.02% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Cancer Biologics Market Trends
“Increasing Focus on Immuno-Oncology Therapies”
One key trend in the cancer biologics market is the increasing focus on immuno-oncology therapies, particularly immune checkpoint inhibitors and CAR T-cell therapies. These treatments are revolutionizing cancer care by harnessing the body’s immune system to target and destroy cancer cells. Immune checkpoint inhibitors, such as PD-1 and PD-L1 inhibitors, have shown significant success in treating cancers such as melanoma, lung, and bladder cancers. Meanwhile, CAR T-cell therapies, which involve genetically modifying a patient's own T-cells to fight cancer, have demonstrated ground-breaking results in treating blood cancers, including leukemia and lymphoma.
This trend is driven by a growing understanding of the immune system's role in cancer progression and the promise of more personalized treatments. As research advances, the potential for expanding these therapies to treat solid tumors is also gaining attention, further fueling the market's growth. The continued success and approval of these therapies are expected to transform cancer treatment for years to come.
Report Scope and Cancer Biologics Market Segmentation
|
Attributes |
Cancer Biologics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
AbbVie Inc. (U.S.), Abbott (U.S.), Accord Healthcare (UK), AstraZeneca (UK), Bayer AG (Germany), Boehringer Ingelheim International GmbH (Germany), Bristol-Myers Squibb Company (U.S.), DAIICHI SANKYO COMPANY, LIMITED (Japan), Dr. Reddy’s Laboratories Ltd. (India), F. Hoffmann-La Roche Ltd (Switzerland), Fresenius Kabi AG (Germany), Genentech, Inc. (U.S.), Gilead Sciences, Inc. (U.S.), GSK plc. (UK), Johnson & Johnson Services, Inc. (U.S.), Merck & Co., Inc. (U.S.), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.), Sanofi (France) and Takeda Pharmaceutical Company Limited (Japan) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cancer Biologics Market Definition
Cancer biologics are a class of biologic drugs used in the treatment of cancer. These therapies are derived from living organisms and are designed to target and attack cancer cells in a more specific and targeted manner compared to traditional chemotherapy. Cancer biologics can include a variety of treatment options, such as monoclonal antibodies, immune checkpoint inhibitors, cancer vaccines, and gene therapies. They work by stimulating the immune system, blocking cancer cell growth, or directly targeting and destroying cancer cells. These therapies have become an essential part of modern cancer treatment, offering more personalized and effective options for patients.
Cancer Biologics Market Dynamics
Drivers
- Advancements in Immuno-Oncology
One of the key drivers of the cancer biologics market is the continuous advancement in immuno-oncology therapies. Treatments such as immune checkpoint inhibitors (for instance, Keytruda, Opdivo) have revolutionized cancer treatment by harnessing the body's immune system to target and kill cancer cells. These therapies have shown remarkable success in treating various cancers, including melanoma, lung cancer, and bladder cancer. In addition, CAR T-cell therapies, such as Kymriah and Yescarta, have made significant strides in treating blood cancers such as leukemia and lymphoma. As research and development in immunotherapy continue to progress, new therapies and combinations are emerging, offering the potential for even greater efficacy and broader applicability across a range of cancers. The growing focus on personalized treatment approaches further drives market expansion, positioning immuno-oncology as a transformative force in cancer care. This growth has significantly impacted the market, driving both innovation and investment in the field.
- Rising Demand for Targeted Therapies
The growing demand for precision medicine and targeted therapies has been a major driver for the cancer biologics market. Targeted therapies, such as monoclonal antibodies and tyrosine kinase inhibitors (TKIs), are designed to specifically attack cancer cells while minimizing damage to normal tissue. Drugs such as Herceptin (trastuzumab) for HER2-positive breast cancer and Gleevec (imatinib) for chronic myelogenous leukemia (CML) are prime instances of how targeted therapies have transformed cancer treatment. These therapies offer more effective treatment options with fewer side effects compared to traditional chemotherapy, leading to improved patient outcomes. With an increasing number of cancer biomarkers being identified, the market for targeted biologics is expanding, particularly for cancers that were once difficult to treat. As more patients seek these precise, tailored therapies, the cancer biologics market is expected to continue growing, driving both market demand and product development in the coming years.
Opportunities
- Expanding Applications for Immuno-Oncology Therapies
A significant opportunity in the cancer biologics market lies in expanding the applications of immuno-oncology therapies to treat a broader range of cancers. While immunotherapies such as checkpoint inhibitors (for instance, Keytruda and Opdivo) have been highly effective for cancers such as melanoma, lung cancer, and bladder cancer, researchers are exploring their potential in treating additional cancer types, such as solid tumors and rare cancers. For instance, trials are underway to test these therapies for cancers such as cervical cancer and triple-negative breast cancer. The ability to expand these therapies to new indications presents substantial market potential. As more cancer types respond to immunotherapy, the market will likely see increased adoption of biologic treatments. This opportunity significantly contributes to market growth by attracting both new patients and investment into ongoing research and development, thereby enhancing the scope and impact of cancer biologics in oncology care.
- Emergence of Personalized Medicine
A major opportunity in the cancer biologics market is the growing demand for personalized or precision medicine. By tailoring treatments to the genetic profile of individual patients and their tumors, cancer biologics can provide more effective and targeted therapies with fewer side effects. For instance, drugs such as Herceptin (trastuzumab) for HER2-positive breast cancer or Kalydeco (ivacaftor) for cystic fibrosis highlight the power of genetic-based treatments. The integration of next-generation sequencing (NGS) technologies allows for the identification of specific genetic mutations, enabling more precise therapies for patients. As personalized medicine becomes increasingly important in oncology, new biologic treatments can be developed that directly target cancer-specific biomarkers. This focus on tailored therapies is driving the development of new biologics and diagnostics, significantly expanding the market for cancer biologics and ensuring their continued growth as more personalized treatment options become available.
Restraints/Challenges
- Safety and Side Effects Concerns
One significant restraint in the cancer biologics market is the potential for severe side effects and safety concerns associated with biologic therapies. While biologic treatments such as immune checkpoint inhibitors (for instance, Keytruda, Opdivo) and CAR T-cell therapies (e.g., Kymriah, Yescarta) have shown remarkable effectiveness, they are also associated with potentially life-threatening side effects. For instance, immune-related adverse events (irAEs), such as severe inflammation or autoimmune responses, have been observed with immunotherapies. CAR T-cell therapies also carry risks such as cytokine release syndrome (CRS) and neurotoxicity, which can be difficult to manage. These safety concerns can limit the use of these therapies in certain patient populations and may result in hesitancy from both physicians and patients to adopt these treatments. As a result, the market for cancer biologics could be restricted if safety concerns are not adequately addressed, impeding broader adoption and slowing growth.
- Manufacturing and Production Complexities
A key challenge for the cancer biologics market is the complexity of manufacturing and production processes, especially for therapies such as CAR T-cell treatments and monoclonal antibodies. These biologics often require specialized facilities, rigorous quality control measures, and highly skilled personnel to produce. For instance, CAR T-cell therapies involve extracting and genetically modifying a patient’s own cells, which is both time-consuming and resource-intensive. The complexity in scaling up production, ensuring consistency, and meeting regulatory standards adds significant costs to the manufacturing process. This challenge can lead to supply shortages and delays in treatment availability, especially as demand grows. The difficulties in manufacturing not only impact the ability to meet market needs promptly but can also affect the pricing of these therapies, ultimately limiting their widespread adoption and hindering overall market growth.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Cancer Biologics Market Scope
The market is segmented on the basis of type of biologic, cancer type, route of administration, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Biologic
- Monoclonal Antibodies
- Vaccines
- Cytokines
- Gene Therapy
- Cell-Based Immunotherapies
- Others
Cancer Type
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Leukemia
- Lymphoma
- Others
Route of Administration
- Intravenous
- Subcutaneous
- Intramuscular
- Others
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Cancer Biologics Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type of biologic, cancer type, route of administration, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the cancer biologics market. This is primarily due to factors such as advanced healthcare infrastructure, significant investments in research and development, and high demand for innovative cancer treatments. The United States, in particular, is home to leading pharmaceutical companies and a large number of clinical trials, which foster rapid development and approval of new biologic therapies. In addition, the high prevalence of cancer, along with favorable reimbursement policies and strong healthcare spending, further supports the growth of the market in this region.
Asia-Pacific is expected to exhibit the highest growth rate in the cancer biologics market. This is due to several factors, including the rapidly increasing cancer incidence, rising healthcare expenditures, and improvements in healthcare infrastructure across key markets such as China, Japan, India, and South Korea. The APAC region is also seeing a rise in the adoption of advanced therapies, such as immunotherapies and targeted treatments, as well as increasing investments from global pharmaceutical companies in expanding their presence.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Cancer Biologics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Cancer Biologics Market Leaders Operating in the Market Are:
- AbbVie Inc. (U.S.)
- Abbott (U.S.)
- Accord Healthcare (UK)
- AstraZeneca (UK)
- Bayer AG (Germany)
- Boehringer Ingelheim International GmbH (Germany)
- Bristol-Myers Squibb Company (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Dr. Reddy’s Laboratories Ltd. (India)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Fresenius Kabi AG (Germany)
- Genentech, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- GSK plc. (UK)
- Johnson & Johnson Services, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
Latest Developments in Cancer Biologics Market
- In February 2025, Bristol Myers Squibb announced that the U.S. Food and Drug Administration (FDA) has accepted the supplemental biologics license application (sBLA) for Opdivo (nivolumab) in combination with Yervoy (ipilimumab) as a potential first-line treatment for adult and pediatric patients (12 years and older) with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer (mCRC). The FDA has granted the application Breakthrough Therapy Designation and Priority Review status, with a Prescription Drug User Fee Act (PDUFA) goal date set for June 23, 2025
- In January 2025, Innovent Biologics, Inc. and Jiangsu Aosaikang Pharmaceutical Co. Ltd. announced that China's National Medical Products Administration (NMPA) has approved the New Drug Application (NDA) for limertinib. This drug is intended for the treatment of adult patients with locally advanced or metastatic EGFR T790M-mutated non-small cell lung cancer (NSCLC). Limertinib marks the 14th product in Innovent's commercial portfolio, further strengthening its tyrosine kinase inhibitor (TKI) franchise and offering a cutting-edge precision therapy for lung cancer patients
- In December 2024, BeiGene, Ltd. announced that the U.S. Food and Drug Administration (FDA) has approved TEVIMBRA (tislelizumab-jsgr), in combination with platinum and fluoropyrimidine-based chemotherapy, for the first-line treatment of unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma (G/GEJ) in adults with tumors expressing PD-L1 (≥1)
- In November 2024, Daiichi Sankyo and AstraZeneca submitted a new Biologics License Application (BLA) for accelerated approval in the U.S. for datopotamab deruxtecan (Dato-DXd). The submission is for the treatment of adult patients with locally advanced or metastatic epidermal growth factor receptor-mutated (EGFR-mutated) non-small cell lung cancer (NSCLC) who have previously received systemic therapies, including an EGFR-targeted treatment
- In September 2024, Takeda announced that it had received approval from the Japanese Ministry of Health, Labour and Welfare to manufacture and market FRUZAQLA Capsules 1mg/5mg (generic name: fruquintinib), a selective oral inhibitor targeting vascular endothelial growth factor receptors (VEGFR) -1, -2, and -3. The drug is approved for the treatment of advanced or recurrent colorectal cancer (CRC) that is not curable or resectable and has progressed following chemotherapy
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.