Global Chimeric Antigen Receptor (CAR)-T Cell Therapy Market, By Types (Abecma, Breyanzi, Kymriah, Tecartus, Yescarta, and Others), Target Antigen (CD 19, CD 20, GD2, CD22, CD30, CD33, HER1, HER2, Meso, Egfrvlll, Others), Therapeutic Application (Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Non-Hodgkin Leukemia, Multiple Myeloma, Pancreatic Cancer, Neuroblasts, Breast Cancer, Acute Myeloid Leukemia, Hepatocellular, Carcinoma, Colorectal Cancer, Others) – Industry Trends and Forecast to 2030.
Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Analysis and Size
The growing number of cancer cases has been shown to increase the chimeric antigen receptor (CAR)-T cell therapy. According to the International Agency for Research on Cancer (IARC), by 2040, cancer cases would increase by 27.5 million cancer diagnoses globally, and cancer death rates will rise by 16.3 million. Additionally, the rise in product approvals by the U.S. FDA makes the market lucrative for investments by the major market players.
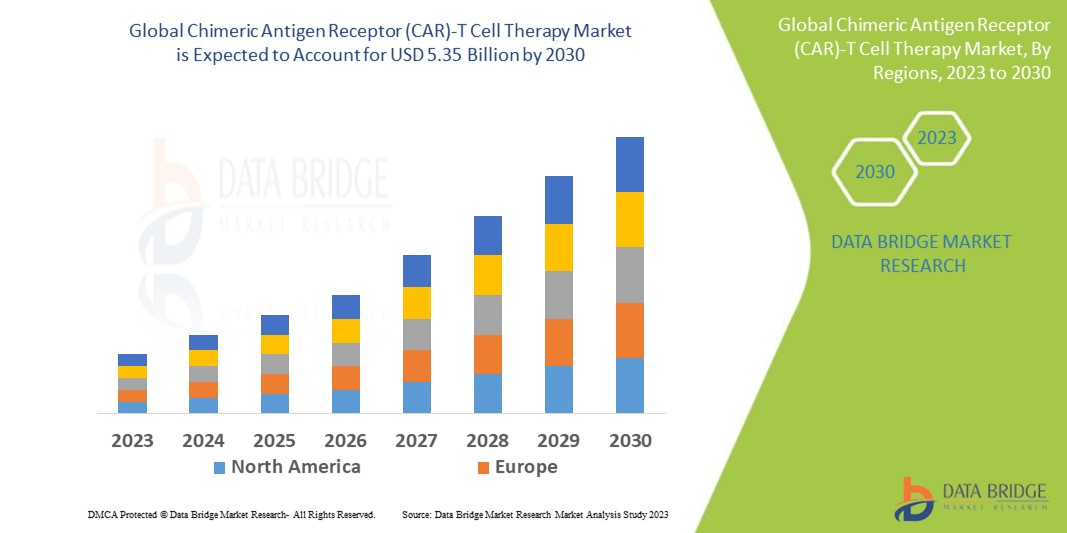
Data Bridge Market Research analyses the growth rate of chimeric antigen receptor (CAR)-T cell therapy in the forecast period 2023-2030. The expected CAGR of the chimeric antigen receptor (CAR)-T cell therapy market is around 11.8% in the mentioned forecast period. The market was valued at USD 2.19 billion in 2022 and would grow to USD 5.35 billion by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Types (Abecma, Breyanzi, Kymriah, Tecartus, Yescarta, and Others), Target Antigen (CD 19, CD 20, GD2, CD22, CD30, CD33, HER1, HER2, Meso, Egfrvlll, Others), Therapeutic Application (Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Non-Hodgkin Leukemia, Multiple Myeloma, Pancreatic Cancer, Neuroblasts, Breast Cancer, Acute Myeloid Leukemia, Hepatocellular, Carcinoma, Colorectal Cancer, Others) |
|
Countries Covered |
U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Autolus Therapeutics (U.K), CARsgen Therapeutics Co.Ltd. (U.K), Juno Therapeutics, Inc.(U.S), Sorrento Therapeutics, Inc. (U.S)., bluebird bio, Inc. (U.S), CELGENE CORPORATION (U.S), Eureka Therapeutics Inc. (U.S), Avacta Life Sciences Ltd. (U.K), Calyxt Inc. (France), Celyad Oncology SA (Belgium), Fortress Biotech, Inc (U.S.)., IMMUNE THERAPEUTICS, INC (U.S.), Gilead Sciences, Inc. (U.S.), Novartis AG (Switzerland), Alaunos Therapeutics, Inc (U.S.)., Poseida Therapeutics, Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Chimeric antigen receptor (CAR)-T cell therapy is a kind of therapy wherein T-cells are taken from the patient with the addition of a special protein receptor on the T-cells that attack cancer cells. This therapy is widely used to treat certain blood cancers and is being studied in treating other types of cancer. Millions of CAR-T cells are grown on a large scale in the laboratory and then given to the patient through an infusion.
Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Dynamics
Drivers
- Increased Prevalence of Cancer
There is a surge in cancer cases globally. The increasing preference for Chimeric antigen receptor (CAR)-T cell therapy amongst healthcare providers suffering from cancer is expected to bolster the market growth. As per the Centers for Disease Control and Prevention (CDCP), in the U.S. in 2019, 1,752,735 new cancer cases were witnessed, and 599,589 people died of cancer. For every 100,000 people, around 439 new cancer cases were recorded, and 146 people died of cancer. The increasing number of cancer cases is letting providers adopt more and more of this therapy due to its multiple benefits, which is expected to boost market growth.
- Growing Product Approvals Associated with Chimeric antigen receptor (CAR)-T cell therapy
The growing number of approvals is also leading to market growth. For instance, the Janssen Pharmaceutical Companies of Johnson & Johnson reported the U.S.FDA approved CARVYKTI in 2022 for the treatment of adults suffering from relapsed or refractory multiple myeloma (RRMM) after four or more prior lines of therapy, which includes an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. Thus, this boosts the market growth
Opportunities
- Increasing Number of Investments by Major Organizations
Increasing investments in chimeric antigen receptor (CAR-T) cell therapy contribute to market growth. For instance, Blackstone stated that funds managed by Blackstone Life Sciences had committed USD 250 million in 2021 to launch a new autologous and allogeneic universal chimeric antigen receptor (CAR) T-cell therapy company. Intellia Therapeutics, Inc. and Cellex Cell Professionals GmbH accompanied this company. Thus, this factor boosts market growth.
- Growing Cases of Multiple Myeloma
The increasing number of cases of multiple myeloma is expected to witness significant growth. For instance, according to GLOBOCAN 2020, the expected number of new cases for multiple myeloma and immunoproliferative diseases in 2020 was around 176,000, and the number is anticipated to reach 290,000 by the year 2040. As per the same source, in 2021, a person was expected to have a 1 in 117 risks of being diagnosed with multiple myeloma by age 85. Thus, this factor helps in the growth of the market.
Restraints/Challenges
- Side-effects of Chimeric antigen receptor (CAR)-T cell therapy
CAR T-Cell therapies have serious side effects such as cancer treatments, such as a mass death of antibody-producing B cells and infections. Cytokine release syndrome (CRS) is witnessed to be one of the most common and severe side effects. T cells release chemical messengers called cytokines that stimulate and direct immune responses as part of immune-related duties. Thus, this factor restraints the market growth.
This chimeric antigen receptor (CAR)-T cell therapy market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the chimeric antigen receptor (CAR)-T cell therapy market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2022, Kite, a Gilead Company, received the U.S.FDA approval of Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients. This therapy is for people suffering from large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
- In 2022, Bristol-Myers Squibb Company stated that Japan's Ministry of Health, Labour and Welfare approved Abecma (idecabtagene violence), which is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell immunotherapy. It treats adult patients suffering from relapsed or refractory (R/R) multiple myeloma.
Global Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Scope
The chimeric antigen receptor (CAR)-T cell therapy market is segmented on the basis of type, target antigen and therapeutic application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Abecma
- Breyanzi
- Kymriah
- Tecartus
- Yescarta
- Others
Target Antigen
- CD 19
- CD 20
- GD2
- CD22
- CD30
- CD33
- HER1
- HER2
- Meso
- Egfrvlll
- Others
Therapeutic Application
- Acute Lymphocytic Leukemia
- Chronic Lymphocytic Leukemia
- Non-Hodgkin Leukemia
- Multiple Myeloma
- Pancreatic Cancer
- Neuroblasts, Breast Cancer
- Acute Myeloid Leukemia
- Hepatocellular
- Carcinoma
- Colorectal Cancer
- Others
Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Regional Analysis/Insights
The chimeric antigen receptor (CAR)-T cell therapy market is analyzed and market size insights and trends are provided by type, target antigen and therapeutic application as referenced above.
The major countries covered in the chimeric antigen receptor (CAR)-T cell therapy market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the market due to robust research and commercial base. Additionally, the increase in clinical trials being conducted for T-cell therapies will further increase the growth of the region's chimeric antigen receptor (CAR)-T cell therapy market during the forecast period.
Asia Pacific is projected to witness huge growth in the market because of the highest number of registered clinical trials associated with the therapies. Additionally, the substantial efforts on government investment and reforms are further estimated to boost the growth of the region's chimeric antigen receptor (CAR)-T cell therapy market in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Chimeric Antigen Receptor (CAR)-T Cell Therapy Market Share Analysis
The chimeric antigen receptor (CAR)-T cell therapy market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related chimeric antigen receptor (CAR)-T cell therapy market.
Key players operating in the chimeric antigen receptor (CAR)-T cell therapy market include:
- Autolus Therapeutics (U.K)
- CARsgen Therapeutics Co.Ltd. (U.K)
- Juno Therapeutics, Inc.(U.S)
- Sorrento Therapeutics, Inc. (U.S)
- bluebird bio, Inc. (U.S)
- CELGENE CORPORATION (U.S)
- Eureka Therapeutics Inc. (U.S)
- Avacta Life Sciences Ltd. (U.K)
- Calyxt Inc.(France)
- Celyad Oncology SA (Belgium)
- Fortress Biotech, Inc (U.S.).
- IMMUNE THERAPEUTICS, INC (U.S.)
- Gilead Sciences, Inc. (U.S.)
- Novartis AG (Switzerland)
- Alaunos Therapeutics, Inc (U.S.).
- Poseida Therapeutics, Inc. (U.S.)
SKU-





 Forecast Period
Forecast Period  Market Size (Base Year)
Market Size (Base Year)  Market Size (Forecast Year)
Market Size (Forecast Year) CAGR
CAGR