Global Car T Cell Therapy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
10.89 Billion
USD
102.21 Billion
2024
2032
USD
10.89 Billion
USD
102.21 Billion
2024
2032
| 2025 –2032 | |
| USD 10.89 Billion | |
| USD 102.21 Billion | |
|
|
|
|
CAR-T Cell Therapy Treatment Market Size
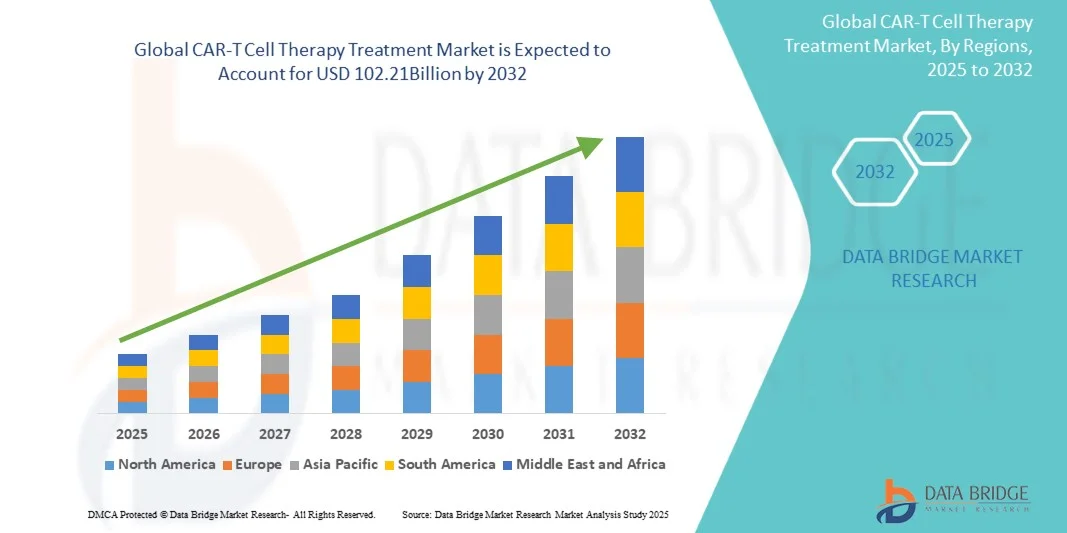
- The global CAR-T Cell Therapy Treatment market size was valued at USD 10.89 billion in 2024 and is expected to reach USD 102.21 billion by 2032, at a CAGR of 32.30% during the forecast period
- The market growth is largely fueled by increasing prevalence of cancer, advancements in immunotherapy research, and the growing adoption of personalized medicine approaches, leading to higher demand for innovative CAR-T cell therapies across clinical settings
- Furthermore, rising patient awareness, improved healthcare infrastructure, and favorable regulatory frameworks are encouraging the development and uptake of CAR-T cell therapy treatments. These converging factors are accelerating the adoption of CAR-T Cell Therapy Treatment solutions, thereby significantly boosting the industry's growth
CAR-T Cell Therapy Treatment Market Analysis
- CAR-T cell therapies, offering advanced immunotherapy solutions for hematologic malignancies and certain solid tumors, are increasingly vital components of modern oncology treatment due to their personalized approach, efficacy, and ability to target cancer cells with precision
- The escalating demand for CAR-T therapies is primarily fueled by rising incidences of blood cancers, growing awareness of innovative treatment options, and advancements in biotechnology enabling safer and more effective therapies
- North America dominated the CAR-T cell therapy treatment market with the largest revenue share of 42.53% in 2024, driven by advanced healthcare infrastructure, high adoption of innovative therapies, and the presence of leading biotechnology and pharmaceutical companies. The U.S. experienced substantial growth due to favorable reimbursement policies, increasing clinical trial activity, and early adoption of CAR-T therapies
- Asia-Pacific is expected to be the fastest-growing region in the CAR-T Cell Therapy Treatment market during the forecast period, fueled by rising healthcare investments, increasing patient awareness, and expanding oncology treatment facilities in countries such as China, Japan, and India
- Hematologic Malignancies dominated the CAR-T cell therapy treatment market with a revenue share of 65% in 2024. The segment’s dominance is driven by established clinical efficacy, strong adoption by hospitals and specialty clinics, and widespread regulatory approvals
Report Scope and CAR-T Cell Therapy Treatment Market Segmentation
|
Attributes |
CAR-T Cell Therapy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
CAR-T Cell Therapy Treatment Market Trends
“Enhanced Convenience Through Advanced Treatment Options”
- A significant and accelerating trend in the global CAR-T Cell Therapy Treatment market is the growing development of innovative, patient-centric therapies that enhance treatment effectiveness and accessibility. These therapies are increasingly designed to improve patient outcomes while minimizing side effects
- For instance, ongoing research is focusing on optimizing CAR-T cell expansion techniques and refining targeting mechanisms to increase efficacy in treating various hematologic malignancies. Several companies are also exploring next-generation CAR-T constructs to address limitations of current therapies
- Advancements in manufacturing processes, including automation and standardized protocols, are enabling faster production and broader distribution of CAR-T treatments, reducing time-to-treatment for patients
- Clinical trials are increasingly exploring combination therapies that integrate CAR-T treatment with other immunotherapies or targeted drugs, aiming to enhance response rates and durability of remission
- Regulatory agencies are providing accelerated approval pathways for promising CAR-T therapies, reflecting their potential to address unmet medical needs and bring treatments to patients more efficiently
- The trend toward personalized CAR-T therapy solutions, tailored to individual patient profiles and tumor characteristics, is reshaping clinical approaches and improving patient management strategies
- Investments in infrastructure and specialized treatment centers are facilitating broader access to CAR-T therapy, particularly in regions where advanced cellular therapy services were previously limited
- The overall demand for CAR-T Cell Therapy Treatment is growing rapidly across both pediatric and adult patient populations, as awareness of its clinical benefits increases and more indications receive approval, driving continued expansion of the market
CAR-T Cell Therapy Treatment Market Dynamics
Driver
“Growing Need Due to Rising Cancer Prevalence and Adoption of Advanced Therapies”
- The increasing prevalence of hematologic malignancies and other cancers, coupled with the accelerating adoption of advanced immunotherapies, is a significant driver for the heightened demand for CAR-T Cell Therapy treatments
- For instance, in April 2024, Kite Pharma (a subsidiary of Gilead Sciences, Inc.) announced a new clinical trial expansion for its next-generation CAR-T therapy targeting multiple B-cell malignancies, highlighting the strategic initiatives by key companies expected to drive the CAR-T Cell Therapy Treatment market growth in the forecast period
- As patients and healthcare providers become more aware of the efficacy of CAR-T therapies in treating refractory and relapsed cancers, demand for these treatments continues to rise. CAR-T therapies offer personalized, targeted treatment options that can achieve remission in patients who have limited alternatives, providing a compelling advantage over conventional therapies
- Furthermore, ongoing advancements in genetic engineering and cellular therapy technologies are making CAR-T treatments safer, more effective, and increasingly accessible, thereby broadening the patient base
- The integration of CAR-T therapies into hospitals, specialty clinics, and research centers, combined with improved reimbursement policies and supportive regulatory frameworks, is a key factor propelling adoption. The trend towards innovative immunotherapies and the increasing availability of user-friendly treatment protocols further contribute to market growth
Restraint/Challenge
“Concerns Regarding High Treatment Costs and Complex Manufacturing Processes”
- The high cost of CAR-T Cell Therapy treatments poses a significant challenge to broader market penetration. As these therapies involve complex, personalized manufacturing processes, treatment costs are substantially higher compared to conventional cancer therapies, limiting accessibility for some patient populations
- For instance, the personalized nature of CAR-T therapy, which requires extraction, genetic modification, and reinfusion of a patient’s own T-cells, contributes to extended production timelines and elevated costs, making it less accessible in developing regions or for price-sensitive patients.
- Addressing these cost challenges through streamlined manufacturing processes, innovative delivery models, and supportive reimbursement policies is crucial for increasing patient access. Companies such as Novartis and Bristol-Myers Squibb are actively investing in process optimization and scalable production techniques to reduce costs and improve affordability
- While treatment efficacy is driving adoption, the complexity of therapy administration, need for specialized healthcare infrastructure, and potential adverse effects still present barriers for wider use
- Overcoming these challenges through enhanced production efficiency, patient education, clinical support services, and financial assistance programs will be vital for sustained growth of the CAR-T Cell Therapy Treatment market
CAR-T Cell Therapy Treatment Market Scope
The market is segmented on the basis of product, structure, targeted antigens, brand, therapeutic application, end-user, and distribution channel.
• By Product
On the basis of product, the CAR-T cell therapy treatment market is segmented into Autologous CAR-T Cells and Allogeneic CAR-T Cells. The autologous CAR-T cells segment dominated the largest market revenue share of 62% in 2024. This dominance is attributed to its personalized approach, proven efficacy in hematologic malignancies, and strong adoption across hospitals and specialty clinics. Clinicians favor autologous therapies due to their established clinical protocols and predictable treatment outcomes. Well-developed manufacturing standards, regulatory approvals, and reimbursement support enhance its market position. Hospitals and specialty clinics trust autologous CAR-T therapies for safety and effectiveness. The segment benefits from strong patient awareness, established distribution networks, and continuous post-market studies. Furthermore, ongoing R&D ensures incremental improvements, reinforcing its leadership globally. The autologous segment’s broad adoption and long-standing clinical reliability make it the cornerstone of the CAR-T therapy market.
The allogeneic CAR-T cells segment is expected to witness the fastest CAGR of 28% from 2025 to 2032. Growth is driven by the off-the-shelf nature of allogeneic therapies, allowing faster treatment initiation and reduced production costs. These therapies are scalable, enabling treatment for a larger patient population, which is fueling adoption in hospitals and specialty clinics. Increasing clinical trials, especially for hematologic and solid tumors, support rapid expansion. Strategic partnerships between biotech companies and hospitals further drive accessibility. Technological advancements in gene editing enhance safety and efficacy, increasing clinician confidence. Rising demand in emerging markets and initiatives to improve therapy availability are contributing to market growth. Regulatory approvals and patient awareness campaigns also play a pivotal role in accelerating the uptake of allogeneic CAR-T cells.
• By Structure
On the basis of structure, the CAR-T cell therapy treatment market is segmented into First Generation, Second Generation, Third Generation, and Fourth Generation CAR-T Cells. The second-generation CAR-T cells dominated with the largest revenue share of 58% in 2024, driven by optimized co-stimulatory domains, enhanced persistence, and strong clinical outcomes. Hospitals and specialty clinics prefer this generation due to its favorable balance of efficacy and safety. Regulatory approvals and well-established clinical protocols support its wide adoption. Continuous improvements in toxicity management and post-infusion monitoring further strengthen its dominance. Second-generation CAR-T therapies have proven success across multiple hematologic malignancies, driving higher prescription rates. Patient outcomes, clinician familiarity, and hospital preference all contribute to robust market leadership. Training programs and post-market data collection enhance confidence and ensure continued adoption globally.
Third and fourth-generation CAR-T cells are expected to witness the fastest CAGR of 27% from 2025 to 2032, fueled by multi-antigen targeting, enhanced tumor infiltration, and improved safety profiles. Growing clinical trial activity for solid tumors accelerates adoption. These advanced generations also support combination therapies, expanding treatment options. Innovations in cell engineering and cost-effective production methods enhance availability. Strategic collaborations between biotech companies and research institutes are fostering development. Expansion of specialty clinics and treatment centers further propels uptake. Continuous regulatory support and R&D investment are expected to sustain rapid growth.
• By Targeted Antigens
On the basis of targeted antigens, the CAR-T cell therapy treatment market is segmented into antigens on solid tumors, antigens on hematologic malignancies, and others. Hematologic malignancy-targeted CAR-T therapies dominated with a revenue share of 65% in 2024. The segment’s dominance is driven by established clinical efficacy, strong adoption by hospitals and specialty clinics, and widespread regulatory approvals. These therapies are used in diseases such as diffuse large B-cell lymphoma, acute lymphoblastic leukemia, and chronic lymphocytic leukemia. Proven safety profiles, reimbursement support, and clinical familiarity enhance adoption. Hospitals and specialty clinics prefer these therapies for predictable outcomes and high success rates. Ongoing monitoring, post-treatment studies, and patient education contribute to sustained growth. The segment is supported by established manufacturing networks and strong supply chains, reinforcing leadership globally.
CAR-T therapies targeting solid tumors are expected to witness the fastest CAGR of 29% from 2025 to 2032, fueled by increasing R&D, clinical trials, and novel antigen discovery. Rising incidence of pancreatic, breast, and lung cancers drives demand. Hospitals and specialty clinics are early adopters of innovative solid tumor therapies. Investment in manufacturing scalability and technology optimization improves access. Patient awareness campaigns and clinician training further accelerate adoption. Expansion into emerging markets and collaborations with research institutions enhance growth. Novel delivery techniques and combination therapies also contribute to the segment’s rapid expansion.
• By Brand
On the basis of brand, the CAR-T cell therapy treatment market is segmented into Yescarta, Kymriah, Tecartus, and others. Yescarta and Kymriah dominated with a combined market share of 55% in 2024, supported by proven clinical efficacy, early market entry, and broad adoption across hospitals and specialty clinics. Strong global distribution networks, clinician familiarity, and post-market surveillance reinforce leadership. Patient trust, positive outcomes, and reimbursement policies further support adoption. Continuous innovation and training programs for hospital staff enhance usage. Brand recognition and established partnerships with healthcare facilities maintain dominance. Ongoing real-world data collection strengthens confidence and encourages continued preference.
Emerging brands are expected to witness the fastest CAGR of 26% from 2025 to 2032, driven by pipeline approvals, regional expansion, and increased investment in production and commercialization. Collaboration with hospitals and specialty clinics enhances market penetration. Focus on novel CAR-T constructs and improved safety profiles accelerates adoption. Regulatory approvals and increased patient awareness support rapid growth. Technological innovation in cell engineering contributes to faster treatment availability. Specialty clinics are adopting emerging brands for solid tumor and combination therapies, driving market expansion.
• By Therapeutic Application
On the basis of therapeutic application, the CAR-T cell therapy treatment market is segmented into hematological malignancies, pancreatic cancer, breast cancer, lung cancer, gastric cancer, multiple myeloma, chronic lymphocytic leukemia, mantle cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, acute lymphoblastic leukemia, and others. Hematological malignancies dominated with a revenue share of 63% in 2024, driven by proven efficacy, strong hospital and specialty clinic adoption, and wide regulatory approval. Established treatment protocols, reimbursement support, and clinical familiarity enhance adoption. Hospitals favor CAR-T therapies for predictable patient outcomes and lower relapse rates. Post-treatment monitoring, safety tracking, and robust supply chains further strengthen the segment. Patient awareness and physician education ensure continued use. Specialty clinics also support adoption through early access programs.
CAR-T therapies for solid tumors are expected to witness the fastest CAGR of 30% from 2025 to 2032, driven by increasing clinical trials, technological innovation, and growing incidence of cancers such as pancreatic, lung, and breast. Hospitals and specialty clinics are early adopters of these innovative therapies. Investment in manufacturing scalability and improved delivery techniques supports growth. Collaborative research and government initiatives promote adoption. Expansion into emerging markets and patient awareness campaigns further boost uptake. Novel CAR-T constructs targeting multiple antigens enhance efficacy and accelerate market penetration.
• By End-User
On the basis of end-user, the CAR-T cell therapy treatment market is segmented into hospitals, specialty clinics, and others. The hospitals segment dominated with a revenue share of 60% in 2024, driven by higher patient volumes, well-established treatment infrastructure, and access to advanced clinical facilities. Hospitals benefit from experienced staff, centralized patient management systems, and established reimbursement frameworks, which ensure predictable adoption and usage. Clinician familiarity with CAR-T protocols, coupled with proven treatment outcomes, reinforces hospital preference. Strong supply chains, hospital pharmacy integration, and post-treatment monitoring capabilities enhance operational efficiency. Patients place high trust in hospital-administered CAR-T therapies due to safety, efficacy, and continuous care support. Awareness campaigns, training programs, and partnerships with key manufacturers further strengthen hospital dominance. Hospitals also serve as referral hubs for specialty clinics, solidifying their leadership in end-user adoption.
The specialty clinics segment is expected to witness the fastest CAGR of 27% from 2025 to 2032, fueled by the rising number of outpatient CAR-T treatment centers and improved accessibility to therapies. Technological innovations, including off-the-shelf allogeneic CAR-T products, are facilitating faster treatment initiation and broader patient reach. Specialty clinics are increasingly expanding in urban and emerging regions to meet growing demand, supported by partnerships with hospitals and research institutes. Enhanced training programs for clinicians and investment in state-of-the-art facilities contribute to higher adoption rates. Patient awareness initiatives, government health programs, and strategic collaborations are driving clinic-based treatments. Rapid adoption is also supported by streamlined operational protocols, cost efficiencies, and improved therapy delivery. The combination of accessibility, convenience, and innovation positions specialty clinics as the fastest-growing end-user segment globally.
• By Distribution Channel
On the basis of distribution channel, the CAR-T cell therapy treatment market is segmented into hospital pharmacies and others. The hospital pharmacy segment dominated with a revenue share of 62% in 2024, benefiting from centralized administration, integrated treatment programs, and established clinician trust. Hospital networks ensure continuous supply, efficient inventory management, and coordinated patient care. Reimbursement support, regulatory approvals, and robust post-treatment follow-ups further strengthen adoption. Hospitals also facilitate patient education, monitoring, and reporting, enhancing overall treatment effectiveness. The presence of experienced pharmacy personnel ensures safe handling and administration of CAR-T therapies. Strong supply chains, established logistics, and partnerships with leading manufacturers solidify the hospital pharmacy segment’s dominance. Hospitals also act as referral points for smaller clinics, further reinforcing leadership in the distribution channel segment.
The other distribution channels segment is expected to witness the fastest CAGR of 28% from 2025 to 2032, driven by the expansion of specialty clinics, growth of off-the-shelf allogeneic CAR-T therapies, and increasing therapy accessibility in emerging regions. Investments in manufacturing capabilities, logistics, and partnerships with research institutes are accelerating supply availability. Early adoption in urban centers and expanding patient awareness are key drivers of growth. Innovative delivery mechanisms and improved cold chain management ensure product integrity and support adoption outside hospitals. Strategic collaborations between manufacturers and specialty clinics enhance penetration. Streamlined procurement processes and growing government initiatives promoting advanced therapies further contribute to rapid expansion. The segment is poised for high growth due to its flexibility, convenience, and ability to serve regions with limited hospital infrastructure.
CAR-T Cell Therapy Treatment Market Regional Analysis
- North America dominated the CAR-T cell therapy treatment market with the largest revenue share of 42.53% in 2024, driven by advanced healthcare infrastructure, high adoption of innovative therapies, and the presence of leading biotechnology and pharmaceutical companies
- The region’s well-established healthcare ecosystem, favorable reimbursement policies, and increasing clinical trial activity have significantly contributed to market growth
- Early adoption of CAR-T therapies, coupled with rising incidence of hematologic malignancies, further supports the demand for advanced cell-based treatments
U.S. CAR-T Cell Therapy Treatment Market Insight
The U.S. CAR-T Cell Therapy treatment market captured the largest revenue share within North America in 2024, fueled by early adoption of CAR-T therapies, expansion of clinical trials, and robust regulatory support. Growing awareness among patients and healthcare providers, combined with the integration of CAR-T therapies into oncology treatment protocols, is driving market expansion. In addition, initiatives by leading biotech companies to enhance therapy efficacy and reduce treatment costs are further propelling growth in the U.S. market.
Europe CAR-T Cell Therapy Treatment Market Insight
The Europe CAR-T Cell Therapy Treatment market is projected to grow at a substantial CAGR during the forecast period, driven by rising healthcare expenditure, increasing prevalence of hematologic cancers, and supportive government policies for advanced therapies. Countries such as Germany, France, and the U.K. are witnessing enhanced adoption due to expanding oncology treatment facilities and improved access to specialized therapies. Increasing investments in research and development for next-generation CAR-T therapies are also contributing to market growth.
U.K. CAR-T Cell Therapy Treatment Market Insight
The U.K. CAR-T Cell Therapy Treatment market is anticipated to expand at a notable CAGR, supported by strong government initiatives to improve cancer care infrastructure and patient access to advanced therapies. Rising clinical trial activity and partnerships between hospitals and biotech companies facilitate broader adoption. The growing awareness of CAR-T therapy benefits among healthcare providers and patients is encouraging increased utilization across both public and private healthcare settings.
Germany CAR-T Cell Therapy Treatment Market Insight
The Germany CAR-T Cell Therapy Treatment market is expected to grow at a considerable CAGR during the forecast period, fueled by well-developed healthcare infrastructure, strong reimbursement frameworks, and the country’s emphasis on innovative cancer treatments. The integration of CAR-T therapies into clinical practice, along with ongoing R&D for improved efficacy and safety, supports the country’s leading position in the European market.
Asia-Pacific CAR-T Cell Therapy Treatment Market Insight
The Asia-Pacific CAR-T Cell Therapy Treatment market is poised to grow at the fastest CAGR during the forecast period, driven by rising healthcare investments, increasing patient awareness, and expanding oncology treatment facilities in countries such as China, Japan, and India. The region benefits from growing government initiatives, improving access to advanced therapies, and the expansion of specialized treatment centers. Rising incidence of hematologic cancers and growing adoption of CAR-T therapies in private and public hospitals are key contributors to rapid market growth.
Japan CAR-T Cell Therapy Treatment Market Insight
The Japan CAR-T cell therapy treatment market is gaining momentum due to government support for advanced therapies, high patient awareness, and increasing clinical trial activity. Expansion of oncology treatment centers, coupled with favorable reimbursement policies, facilitates the adoption of CAR-T therapies. Moreover, ongoing collaborations between local hospitals and biotech companies are driving the introduction of next-generation CAR-T therapies.
China CAR-T Cell Therapy Treatment Market Insight
The China CAR-T cell therapy treatment market accounted for the largest revenue share in Asia-Pacific in 2024, driven by increasing healthcare investments, rising patient awareness, and the rapid expansion of oncology treatment facilities. The market benefits from government initiatives promoting advanced therapies, the growing number of specialized treatment centers, and partnerships between domestic and global biotech companies. These factors are accelerating CAR-T therapy adoption across both urban and semi-urban healthcare settings.
CAR-T Cell Therapy Treatment Market Share
The CAR-T Cell Therapy Treatment industry is primarily led by well-established companies, including:
- Autolus Therapeutics (U.K.)
- Atara Biotherapeutics, Inc. (U.S.)
- Amgen Inc. (U.S.)
- Bellicum Pharmaceuticals, Inc. (U.S.)
- bluebird bio, Inc. (U.S.)
- Adaptimmune (U.K.)
- Bristol-Myers Squibb Company (U.S.)
- Xyphos Biosciences, Inc. (U.S.)
- Johnson & Johnson and its affiliates (U.S.)
- BioAtla Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Novartis AG (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- Cartherics Pty. Ltd (Australia)
- CARINA BIOTECH (Australia)
- Alaunos Therapeutics, Inc. (U.S.)
- Cellectis SA (France)
- Mustang Bio (U.S.)
- Sorrento Therapeutics, Inc. (U.S.)
- Cartesian Therapeutics, Inc. (U.S.)
- TC BioPharm Limited (U.K.)
- Celyad Oncology SA (Belgium)
Latest Developments in CAR-T Cell Therapy Treatment Market
- In June 2025, a dual-target CAR-T cell therapy approach demonstrated promising results in slowing tumor growth in patients with aggressive brain cancer. Nearly two-thirds of participants experienced tumor shrinkage, highlighting the potential of this strategy in treating challenging malignancies
- In June 2025, a phase 1 clinical trial initiated the dosing of the first patient with a switchable CAR-T cell therapy for metastatic breast cancer. This innovative approach aims to enhance the safety and efficacy of CAR-T therapies by providing controllable activation, offering a new avenue for treating solid tumors
- In May 2025, a groundbreaking immunotherapy trial demonstrated that CAR-T cell therapy could significantly extend the survival of patients with advanced gastric or gastro-oesophageal junction cancers. Patients receiving CAR-T therapy lived on average 7.9 months post-treatment, compared to 5.5 months with standard care, marking a 40% improvement in survival rates
- In April 2025, researchers at the University of Colorado Anschutz Medical Campus developed a supercharged version of CAR-T cell therapy, known as ALA-CART. This enhanced therapy showed increased effectiveness and longevity in targeting hard-to-treat cancer cells, particularly in cases where previous CAR-T therapies were less effective
- In January 2025, the U.S. Food and Drug Administration (FDA) declined to approve Atara Biotherapeutics' cancer therapy, tabelecleucel, for patients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease (EBV+ PTLD). The rejection was based on issues found during an inspection of a third-party manufacturing facility. Atara plans to resubmit for approval after addressing the manufacturing compliance issues
- In August 2024, the FDA announced an investigation into more than 20 instances of secondary T-cell cancers, specifically T-cell lymphomas, in individuals treated with CAR-T cell therapies. In some cases, genes used to make the CAR-T treatments were present in the secondary cancers, raising concerns about the long-term safety of these therapies
- In June 2023, Nagpur became the first city in central India to offer advanced CAR-T cell therapy for blood cancer. Two patients with relapsed Acute Lymphoblastic Leukaemia (ALL) were successfully treated, marking a significant milestone in making cutting-edge cancer treatments accessible in tier-2 cities
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.