Global Cardiac Resynchronization Therapy Crt Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
7.94 Billion
USD
11.68 Billion
2025
2033
USD
7.94 Billion
USD
11.68 Billion
2025
2033
| 2026 –2033 | |
| USD 7.94 Billion | |
| USD 11.68 Billion | |
|
|
|
|
Cardiac Resynchronization Therapy (CRT) Devices Market Size
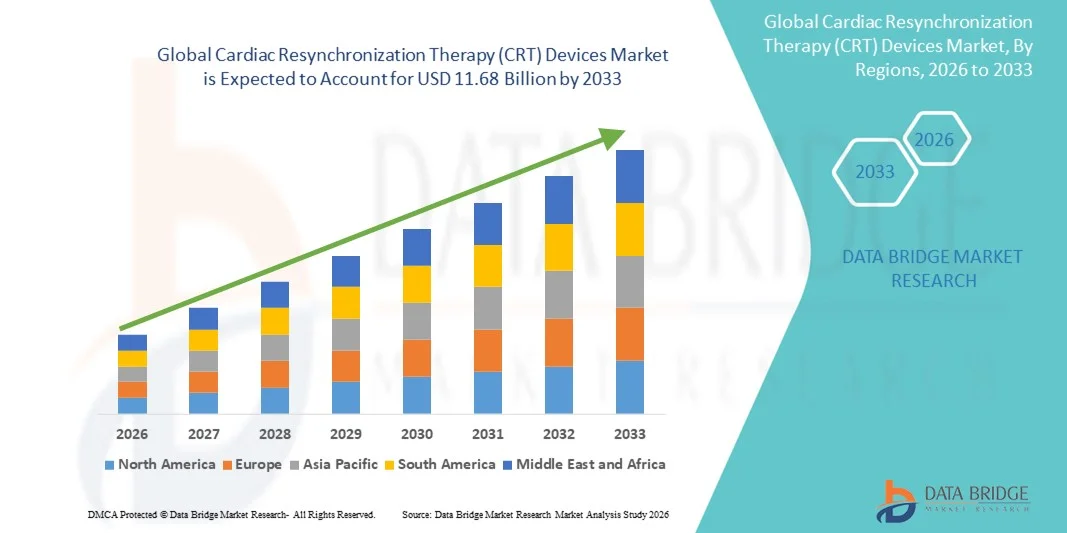
- The global cardiac resynchronization therapy (CRT) devices market size was valued at USD 7.94 billion in 2025 and is expected to reach USD 11.68 billion by 2033, at a CAGR of 4.95% during the forecast period
- The escalating prevalence of heart failure, coupled with technological advancements in implantable cardiac devices, is driving the growth of the Cardiac Resynchronization Therapy (CRT) Devices market. CRT devices are increasingly being adopted for patients with advanced heart failure to improve cardiac function, quality of life, and reduce hospitalization rates
- Furthermore, rising awareness among clinicians and patients regarding the clinical benefits of CRT, along with improved device features such as MRI compatibility, extended battery life, and remote monitoring capabilities, is accelerating market adoption. These factors are significantly contributing to the market’s expansion
Cardiac Resynchronization Therapy (CRT) Devices Market Analysis
- Cardiac Resynchronization Therapy (CRT) Devices, providing synchronized pacing for patients with heart failure and conduction abnormalities, are increasingly vital in improving cardiac function, reducing hospitalizations, and enhancing quality of life for patients across advanced healthcare systems globally
- The escalating demand for CRT Devices is primarily fueled by the rising prevalence of heart failure, increasing awareness among clinicians and patients, technological advancements in implantable devices, and supportive reimbursement policies in developed and emerging regions
- North America dominated the cardiac resynchronization therapy (CRT) devices market with the largest revenue share of approximately 42% in 2025, supported by advanced healthcare infrastructure, strong reimbursement policies, high prevalence of heart failure, and the presence of major device manufacturers. The U.S. accounted for the majority of regional demand due to established cardiac care networks, increasing adoption of implantable devices, and continuous investments in cardiac treatment programs
- Asia-Pacific is expected to be the fastest-growing region in the cardiac resynchronization therapy (CRT) Devices market during the forecast period, registering a CAGR of around 11.8% from 2026 to 2033. Growth in this region is driven by increasing cardiovascular disease prevalence, improving healthcare infrastructure, rising awareness of advanced cardiac therapies, and expanding access to specialized cardiac care facilities in countries such as China, India, and Japan
- The CRT-Pacemakers segment dominated the largest market revenue share of 57.8% in 2025, driven by their widespread adoption for patients with moderate to severe heart failure and left ventricular dysfunction
Report Scope and Cardiac Resynchronization Therapy (CRT) Devices Market Segmentation
|
Attributes |
Cardiac Resynchronization Therapy (CRT) Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Medtronic (Ireland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cardiac Resynchronization Therapy (CRT) Devices Market Trends
Growing Prevalence of Heart Failure and Cardiovascular Disorders
- The rising global prevalence of heart failure and other cardiovascular disorders is a significant driver for the increased adoption of CRT devices. The World Health Organization reports that cardiovascular diseases remain the leading cause of death globally, emphasizing the need for advanced therapeutic interventions
- CRT devices improve cardiac output, reduce symptoms of heart failure, and enhance patient quality of life, making them a preferred treatment for eligible patients
- For instance, in March 2024, Medtronic expanded its CRT-D device portfolio to include the Gallant™ CRT-D system with improved battery longevity and adaptive pacing features, aimed at patients with advanced heart failure
- Advancements in device miniaturization and lead design have improved implantation success rates, further supporting market growth
- Increasing awareness among cardiologists and patients about the benefits of CRT devices is driving higher adoption rates in both developed and emerging markets
- Integration with remote monitoring platforms allows clinicians to track patient response, optimize therapy, and reduce hospitalization, strengthening the market demand
- Growing government initiatives and reimbursement policies for cardiovascular interventions in countries like the U.S., Germany, and Japan are positively influencing the market
- Developing countries are witnessing expansion of tertiary cardiac care centers, improving access to CRT devices for patients previously underserved
- The combination of clinical efficacy, improved patient outcomes, and supportive healthcare infrastructure continues to propel CRT device adoption globally
- The market is further boosted by partnerships between device manufacturers and hospitals for training and awareness programs on CRT therapy
- New product launches with extended battery life and MRI compatibility also encourage adoption among physicians
- Overall, the rising incidence of heart failure coupled with technological advancements in CRT devices is expected to remain a key growth driver through the forecast period
Cardiac Resynchronization Therapy (CRT) Devices Market Dynamics
Driver
Increasing Adoption of Remote Patient Monitoring and Telehealth Integration
- A notable trend in the CRT devices market is the increasing incorporation of remote patient monitoring (RPM) and telehealth technologies, allowing continuous evaluation of patient cardiac function
- For instance, in January 2025, Abbott received regulatory clearance for the Confirm™ CRT-D system with enhanced remote monitoring capabilities, enabling clinicians to track device performance and patient heart rhythms remotely
- Remote monitoring helps reduce hospital visits, improves therapy compliance, and allows timely interventions in case of complications. Integration with telemedicine platforms facilitates patient follow-ups without the need for in-person consultations, enhancing convenience for both patients and healthcare providers
- Physician dashboards provide real-time analytics on device performance, enabling proactive treatment adjustments. The trend is particularly gaining traction in North America and Europe, where healthcare systems are emphasizing value-based care and reducing hospitalization costs
- In emerging markets, adoption is increasing gradually as infrastructure and reimbursement mechanisms improve. Advanced CRT devices now allow automatic detection of arrhythmias and heart failure exacerbations, triggering alerts for clinicians via remote platforms
- Healthcare providers are increasingly leveraging cloud-based solutions to monitor multiple patients simultaneously, enhancing workflow efficiency. Patient engagement apps linked to CRT devices improve adherence and provide educational resources about heart failure management
- The combination of RPM and telehealth integration is shaping a patient-centric approach in cardiac care and expanding CRT device utilization globally. As technology adoption grows, this trend is expected to continue accelerating market growth over the forecast period
Restraint/Challenge
High Device Cost and Procedural Complexity
- The relatively high cost of CRT devices and implantation procedures poses a significant challenge for market growth, particularly in price-sensitive regions
- For instance, CRT device systems can range from USD 20,000 to USD 40,000, with additional procedural and hospitalization costs, which limits accessibility in developing countries
- Complex implantation procedures requiring specialized training and skilled electrophysiologists can restrict adoption in smaller or rural hospitals
- Potential procedural complications, such as lead dislodgement, infection, or pneumothorax, increase hesitancy among both patients and healthcare providers
- Limited reimbursement coverage in some emerging markets further constrains market penetration. Lengthy implantation procedures and post-operative monitoring requirements add operational burden to healthcare facilities
- Device recalls or adverse events reported in clinical studies can negatively impact physician confidence and patient acceptance. Concerns about battery replacement surgeries and device longevity also affect long-term adoption
- Efforts to reduce device costs through innovations and government incentives are ongoing but have yet to achieve universal affordability. Physician training programs and procedural standardization are critical to overcome procedural complexity barrier
- Despite clinical efficacy, the high upfront investment and operational challenges remain key market restraints. Addressing cost concerns, improving procedure efficiency, and expanding reimbursement coverage will be essential to sustain CRT device market growth globally
Cardiac Resynchronization Therapy (CRT) Devices Market Scope
The market is segmented on the basis of product type and end-user.
- By Product Type
On the basis of product type, the Cardiac Resynchronization Therapy (CRT) Devices market is segmented into CRT-Pacemakers and CRT-Defibrillators. The CRT-Pacemakers segment dominated the largest market revenue share of 57.8% in 2025, driven by their widespread adoption for patients with moderate to severe heart failure and left ventricular dysfunction. CRT-Pacemakers are often preferred due to their comparatively lower cost, ease of implantation, and established clinical efficacy in improving cardiac output and patient quality of life. The segment benefits from well-established clinical guidelines recommending CRT-Pacemakers for eligible heart failure patients. Hospitals and cardiac specialty clinics prefer CRT-Pacemakers for their lower complication rates and proven long-term outcomes. Growing awareness of heart failure management among patients and physicians further reinforces demand. Product advancements such as battery longevity improvements, MRI compatibility, and miniaturized designs enhance adoption. Reimbursement coverage in developed countries supports high penetration. Clinical studies demonstrating symptom improvement and reduction in hospitalization also drive market preference. The CRT-Pacemakers segment’s dominance is further strengthened by ongoing physician training programs and hospital collaborations for device implementation. Overall, strong clinical trust, guideline-backed usage, and increasing prevalence of heart failure patients sustain CRT-Pacemakers’ market leadership.
The CRT-Defibrillators segment is anticipated to witness the fastest CAGR of 14.2% from 2026 to 2033, driven by rising demand for devices that combine resynchronization therapy with life-saving defibrillation capabilities. These devices are preferred for high-risk heart failure patients prone to sudden cardiac arrest. Technological advancements such as remote monitoring, multi-site pacing, and enhanced arrhythmia detection further fuel growth. Growing awareness among cardiologists about the preventive benefits of CRT-Defibrillators accelerates adoption. Emerging markets are gradually increasing access through improved healthcare infrastructure. Insurance coverage expansion in developed countries makes CRT-Defibrillators more accessible. Clinical guidelines increasingly recommend CRT-Defibrillators for patients with severe ventricular arrhythmias. Rising prevalence of ischemic cardiomyopathy and ventricular tachyarrhythmias supports market growth. Device miniaturization and reduced procedural complexity improve patient acceptance. Hospitals and specialized clinics are investing in advanced CRT-Defibrillator implantation programs. Continuous R&D ensures better safety and efficacy, further encouraging usage. High physician confidence in device outcomes and growing patient awareness are key drivers of this rapid CAGR.
- By End-User
On the basis of end-user, the Cardiac Resynchronization Therapy (CRT) Devices market is segmented into clinics and ambulatory surgical centres. The clinics segment accounted for the largest market revenue share of 61% in 2025, owing to their established network of cardiologists, availability of trained staff, and infrastructure for routine implantation and follow-up of CRT devices. Clinics often provide comprehensive cardiac care, including pre-procedural assessments, device implantation, and post-operative monitoring. The segment benefits from strong referral networks and trusted relationships with hospitals. Adoption is further supported by reimbursement policies and clinical guidelines recommending CRT device implantation in outpatient clinic settings. Clinics also have better access to advanced diagnostic tools and electrophysiology labs. Physician expertise in clinics ensures high procedural success rates and patient confidence. The segment’s dominance is strengthened by ongoing education programs for cardiologists and technical staff. Partnerships with device manufacturers enhance product availability and training. Clinics provide personalized care and long-term monitoring, which is a critical factor driving revenue share. Established healthcare systems in North America and Europe support continued growth in this segment. Access to patient management systems and electronic health records further facilitates CRT adoption in clinic settings.
The ambulatory surgical centres segment is expected to witness the fastest CAGR of 12.8% from 2026 to 2033, driven by the shift toward cost-effective and specialized cardiac care facilities. Improvements in minimally invasive implantation techniques make outpatient procedures more viable. Shorter recovery times and reduced hospital stays fuel adoption. Growing investments in ambulatory surgical infrastructure support growth. Rising patient preference for convenience and reduced hospitalization costs contributes to expansion. Increasing collaboration between device manufacturers and surgical centers enhances availability. Ambulatory centres often serve patients in semi-urban and urban areas, improving accessibility. Expansion of cardiac specialty centers globally supports adoption. Advanced training programs for electrophysiologists in outpatient settings encourage procedural confidence. Regulatory approvals and accreditation of ambulatory surgical centres further drive market penetration. Cost-effectiveness combined with improved patient experience strengthens the growth trajectory. Increasing demand for same-day procedures in high-volume centers accelerates the CAGR. Overall, ambulatory surgical centres are becoming key players in CRT device deployment.
Cardiac Resynchronization Therapy (CRT) Devices Market Regional Analysis
- North America dominated the cardiac resynchronization therapy (CRT) devices market with the largest revenue share of approximately 42% in 2025, supported by advanced healthcare infrastructure, strong reimbursement policies, high prevalence of heart failure, and the presence of major device manufacturers
- The market accounted for the majority of regional demand due to established cardiac care networks, increasing adoption of implantable devices, and continuous investments in cardiac treatment programs. Moreover, growing awareness among physicians and patients regarding early diagnosis and treatment of heart failure is contributing to market growth
- The presence of key players such as Medtronic, Boston Scientific, and Abbott further strengthens the regional market. Increasing clinical trials, advancements in CRT-P and CRT-D devices, and initiatives to improve patient outcomes are anticipated to sustain market dominance
U.S. Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The U.S. cardiac resynchronization therapy (CRT) devices market captured the largest revenue share within North America in 2025, driven by the rapid adoption of implantable CRT systems and well-established cardiac care programs. High prevalence of cardiovascular diseases, advanced hospital infrastructure, and favorable reimbursement policies encourage widespread utilization. Ongoing investments in device innovations, such as improved battery life and miniaturized CRT-P devices, enhance patient compliance and outcomes. Additionally, government initiatives promoting cardiovascular health awareness and early intervention programs are expected to further propel market expansion.
Europe Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The Europe cardiac resynchronization therapy (CRT) devices market is projected to expand at a substantial CAGR throughout the forecast period, driven by increasing prevalence of heart failure, rising geriatric population, and advanced healthcare services. Germany, France, and the U.K. are key contributors due to well-established cardiac care networks and high adoption of implantable CRT devices. Increasing awareness programs, stringent clinical guidelines, and growing healthcare expenditure on advanced therapies are supporting the regional market. Expanding collaborations between device manufacturers and hospitals further enhance access to CRT devices across Europe.
U.K. Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The U.K. cardiac resynchronization therapy (CRT) devices market is expected to grow at a noteworthy CAGR during the forecast period, fueled by rising heart failure prevalence, increased geriatric population, and adoption of advanced cardiac therapies. National Health Service (NHS) initiatives focusing on cardiovascular disease management and early interventions are supporting market growth. Moreover, the expansion of specialized cardiac centers and cardiac implant programs in hospitals encourages wider adoption of CRT devices. The presence of leading manufacturers and continuous clinical research are further driving regional market development.
Germany Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The Germany cardiac resynchronization therapy (CRT) devices market is anticipated to expand at a considerable CAGR during the forecast period, driven by the rising prevalence of heart failure, advanced healthcare infrastructure, and strong emphasis on cardiac care innovation. Hospitals are increasingly adopting CRT-P and CRT-D devices to improve patient outcomes. Government support for healthcare modernization and initiatives promoting early diagnosis of cardiovascular diseases further support growth. Collaborations between local cardiac centers and device manufacturers are enhancing accessibility and distribution of CRT therapies in Germany.
Asia-Pacific Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The Asia-Pacific cardiac resynchronization therapy (CRT) devices market is expected to register the fastest CAGR of 11.8% from 2026 to 2033, driven by increasing prevalence of cardiovascular diseases, improving healthcare infrastructure, and rising awareness of advanced cardiac therapies. Growth is concentrated in countries such as China, India, and Japan, where expanding access to specialized cardiac care facilities and increasing investments in healthcare technology support market expansion. The growing geriatric population, rising disposable incomes, and government initiatives promoting cardiovascular health are further fueling adoption. Continuous efforts to enhance patient access to implantable CRT devices and rising cardiac treatment programs contribute to the rapid growth of this regional market.
Japan Cardiac Resynchronization Therapy (CRT) Devices Market Insight
The Japan cardiac resynchronization therapy (CRT) devices market is growing steadily due to high prevalence of heart failure, aging population, and increasing demand for advanced cardiac therapies. Hospitals and specialized cardiac centers are investing in state-of-the-art CRT-P and CRT-D devices. Government-led initiatives to improve cardiac care infrastructure, along with favorable reimbursement policies, are supporting market expansion. Additionally, ongoing clinical trials and adoption of innovative device technologies further drive growth.
China Cardiac Resynchronization Therapy (CRT) Devices Market Insight
China cardiac resynchronization therapy (CRT) devices market accounted for the largest share of the Asia-Pacific CRT devices market in 2025, driven by the rising burden of cardiovascular diseases, large patient population, and increasing healthcare expenditure. Rapid urbanization, improved healthcare infrastructure, and government support for cardiovascular treatment programs facilitate higher adoption of CRT devices. Local device manufacturing, expansion of specialized cardiac centers, and awareness programs targeting early diagnosis and treatment of heart failure further support market growth.
Cardiac Resynchronization Therapy (CRT) Devices Market Share
The Cardiac Resynchronization Therapy (CRT) Devices industry is primarily led by well-established companies, including:
• Medtronic (Ireland)
• Boston Scientific (U.S.)
• Abbott (U.S.)
• Cordis (U.S.)
• Biotronik (Germany)
• Sorin Group (Italy)
• LivaNova (U.K.)
• MicroPort CRM (China)
• Shenzhen Mindray Bio-Medical Electronics (China)
• AliveCor (U.S.)
• GE Healthcare (U.S.)
• Philips Healthcare (Netherlands)
• Sequent Medical (U.S.)
• Carmat (France)
• HeartWare International (U.S.)
• Implandata Ophthalmic Products (Germany)
• Neovasc Inc. (Canada)
• Veloxis Pharmaceuticals (Denmark)
• Stimwave Technologies (U.S.)
• Virtuozo Medical (Brazil)
Latest Developments in Global Cardiac Resynchronization Therapy (CRT) Devices Market
- In July 2023, BIOTRONIK obtained U.S. regulatory approval for its Amvia Edge pacemaker / CRT‑P platform — a compact, MRI‑conditional CRT‑pacemaker family with an automatic MRI‑detection algorithm (“MRI Guard 24/7”). This makes MRI scans easier and safer for CRT patients, potentially reducing complications and broadening CRT‑P adoption
- In February 2024, MicroPort Scientific Corporation launched a new CRT‑D system (with its GALI SonR CRT and NAVIGO 4LV lead) in Japan. The new system features a hemodynamic sensor that automatically optimizes pacing delays — which has been associated with lower heart‑failure hospitalizations and more effective resynchronization therapy
- In September 2025, Biotronik announced the first‑in‑human implantation of its new CRT‑D device engineered for left‑bundle branch area pacing (LBBAP), aiming to offer more physiologic ventricular activation than conventional biventricular pacing. This marks a major advance in CRT device design, potentially expanding therapy options for heart‑failure patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.