Global Cardiopulmonary Resuscitation Crp Devices Market
Market Size in USD Million
CAGR :
% 
 USD
237.66 Million
USD
538.02 Million
2025
2033
USD
237.66 Million
USD
538.02 Million
2025
2033
| 2026 –2033 | |
| USD 237.66 Million | |
| USD 538.02 Million | |
|
|
|
|
Cardiopulmonary Resuscitation (CRP) Devices Market Size
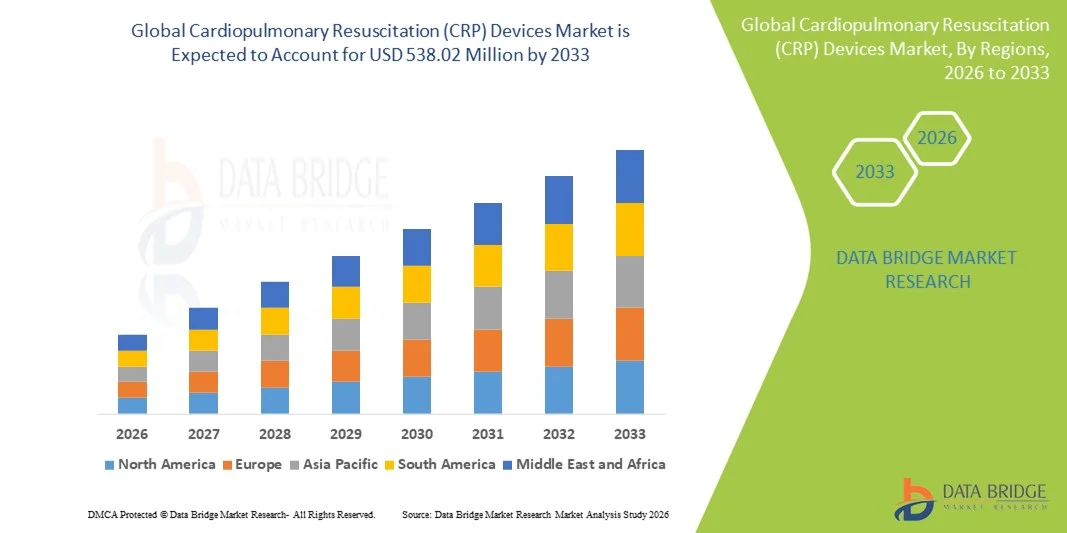
- The global Cardiopulmonary Resuscitation (CRP) devices market size was valued at USD 237.66 million in 2025 and is expected to reach USD 538.02 million by 2033, at a CAGR of 10.76% during the forecast period
- The market growth is primarily driven by the rising prevalence of sudden cardiac arrest, increasing incidence of cardiovascular diseases, and growing awareness regarding the importance of timely and effective resuscitation in both in-hospital and out-of-hospital settings
- Furthermore, continuous technological advancements in automated and mechanical CPR devices, along with expanding adoption by emergency medical services, hospitals, and public access programs, are establishing CPR devices as critical life-saving tools, thereby significantly boosting the overall market growth
Cardiopulmonary Resuscitation (CRP) Devices Market Analysis
- Cardiopulmonary resuscitation (CPR) devices, encompassing advanced mechanical and adjunctive technologies designed to enhance blood circulation during cardiac arrest, are becoming indispensable across emergency and critical care settings due to their ability to deliver controlled, guideline-compliant resuscitation support and improve patient survival outcomes
- The increasing demand for CPR devices is mainly driven by the rising global burden of cardiovascular diseases, growing incidence of sudden cardiac arrest, and expanding emphasis on improving resuscitation efficiency in both in-hospital and pre-hospital emergency care environments
- North America dominated the CPR devices market with the largest revenue share of 41.0% in 2025, supported by advanced emergency response infrastructure, high adoption of innovative resuscitation technologies, and strong presence of leading manufacturers, with the U.S. showing extensive utilization across emergency departments and coronary & intensive care units
- Asia-Pacific is anticipated to be the fastest-growing region during the forecast period, owing to rapid improvements in healthcare infrastructure, increasing investments in emergency medical services, and rising awareness regarding advanced life-support technologies across developing economies
- Mechanical piston devices segment dominated the market with a share of 38.5% in 2025, driven by their widespread adoption in emergency departments and air medevac units, ability to deliver consistent chest compressions, and growing preference for automated solutions in high-acuity resuscitation scenarios
Report Scope and Cardiopulmonary Resuscitation (CRP) Devices Market Segmentation
|
Attributes |
Cardiopulmonary Resuscitation (CRP) Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cardiopulmonary Resuscitation (CRP) Devices Market Trends
Shift Toward Automated and Guideline-Compliant Resuscitation Technologies
- A significant and accelerating trend in the global CPR devices market is the increasing adoption of automated and mechanical resuscitation systems that ensure consistent, guideline-compliant chest compressions across both in-hospital and pre-hospital settings
- For instance, mechanical piston and load-distributing band CPR devices are increasingly deployed by emergency medical services (EMS) to maintain uninterrupted compressions during patient transport, addressing limitations of manual CPR
- Technological advancements in CPR devices are enabling real-time feedback, adjustable compression depth, and integration with defibrillators, improving clinical outcomes and adherence to international resuscitation guidelines
- The growing use of impedance threshold devices and active compression-decompression (ACD) technologies is enhancing venous return and perfusion during cardiac arrest, reflecting a broader shift toward physiology-driven resuscitation
- This trend toward more precise, automated, and performance-optimized CPR solutions is reshaping expectations among clinicians, prompting manufacturers to focus on usability, portability, and compatibility with advanced life-support systems
- The demand for advanced CPR devices is rising steadily across emergency departments, coronary & intensive care units, and air medevac units as healthcare providers prioritize high-quality resuscitation and improved survival rates
- Increasing miniaturization and portability of CPR devices is supporting their wider adoption in air medevac units and remote emergency response scenarios
Cardiopulmonary Resuscitation (CRP) Devices Market Dynamics
Driver
Rising Incidence of Cardiac Arrest and Emphasis on Emergency Care Outcomes
- The increasing global incidence of sudden cardiac arrest, coupled with the growing burden of cardiovascular diseases, is a major driver fueling demand for advanced CPR devices
- For instance, healthcare systems are increasingly investing in mechanical CPR and extracorporeal resuscitation technologies to improve survival rates in refractory cardiac arrest cases
- As awareness of the importance of early and high-quality resuscitation increases, hospitals and emergency responders are adopting CPR devices to reduce variability and fatigue associated with manual chest compressions
- Furthermore, expanding emergency medical services infrastructure and improved access to critical care facilities are supporting higher adoption of CPR devices across both urban and semi-urban regions
- The growing focus on standardized emergency care protocols and outcome-based healthcare delivery is reinforcing the role of CPR devices as essential tools in modern resuscitation practices
- Increasing government initiatives and funding for emergency preparedness programs are accelerating procurement of CPR devices by public healthcare institutions
- Growing deployment of CPR devices in organ transplant facilities is supporting better organ perfusion outcomes, further driving market demand
Restraint/Challenge
High Device Cost and Training-Intensive Deployment Requirements
- The high cost associated with advanced CPR devices, particularly mechanical and extracorporeal systems, poses a significant challenge to widespread adoption, especially in resource-constrained healthcare settings
- For instance, smaller hospitals and emergency units may hesitate to invest in load-distributing band or invasive perfusion devices due to budget limitations and competing capital expenditure priorities
- The effective use of CPR devices often requires specialized training and regular skill refreshers, creating operational and logistical challenges for healthcare providers
- In addition, device maintenance requirements and the need for regulatory approvals across different regions can delay procurement and deployment timelines
- Addressing these challenges through cost-effective device innovation, expanded training programs, and supportive reimbursement policies will be critical for sustained growth of the CPR devices market
- Limited reimbursement coverage for advanced CPR procedures in certain regions can restrict hospital purchasing decisions
- Resistance to replacing traditional manual CPR techniques among some clinical teams can slow adoption despite proven device efficacy
Cardiopulmonary Resuscitation (CRP) Devices Market Scope
The market is segmented on the basis of device type, application, and end user.
- By Device Type
On the basis of device type, the cardiopulmonary resuscitation (CPR) devices market is segmented into mechanical piston devices, active compression-decompression (ACD) devices, impedance threshold devices, load-distributing band CPR or vest CPR, phased thoracic-abdominal compression-decompression CPR with a hand-held device, extracorporeal techniques, and invasive perfusion devices. The mechanical piston devices segment dominated the market with the largest revenue share of 38.5% in 2025, driven by their widespread adoption across emergency departments and pre-hospital emergency medical services. These devices deliver consistent, guideline-compliant chest compressions, reducing rescuer fatigue and minimizing variability associated with manual CPR. Their portability and ease of deployment make them particularly valuable in ambulances and air medevac units. In addition, strong clinical familiarity and long-standing use in advanced life support protocols further support their dominant position. Hospitals prefer mechanical piston devices due to proven reliability in prolonged resuscitation scenarios.
The extracorporeal techniques segment is expected to witness the fastest growth during the forecast period, supported by increasing adoption of extracorporeal cardiopulmonary resuscitation (ECPR) in advanced cardiac care centers. These systems provide temporary circulatory and respiratory support in refractory cardiac arrest cases where conventional CPR fails. Growing investments in ECMO-capable facilities and rising evidence supporting improved neurological outcomes are accelerating adoption. Tertiary hospitals and academic medical centers are increasingly integrating extracorporeal techniques into cardiac arrest management protocols. The expansion of specialized cardiac units globally further contributes to the rapid growth of this segment.
- By Application
On the basis of application, the CPR devices market is segmented into emergency departments, coronary & intensive care units, organ transplant facilities, air medevac units, and EMT rescue units. The emergency departments segment accounted for the largest market share in 2025, driven by the high incidence of sudden cardiac arrest cases presenting in hospital emergency settings. Emergency departments require rapid, reliable, and standardized resuscitation solutions, making mechanical and automated CPR devices essential. The availability of trained personnel and advanced life-support infrastructure further supports higher device utilization. Continuous patient inflow and the need for uninterrupted CPR during diagnostics also reinforce demand. Emergency departments remain the primary point of deployment for CPR devices globally.
The air medevac units segment is anticipated to grow at the fastest rate over the forecast period due to increasing reliance on rapid medical transport for critically ill cardiac patients. Delivering manual CPR during air transport is challenging due to space constraints and safety concerns, driving demand for automated CPR devices. Growing investments in helicopter emergency medical services and long-distance critical care transport are supporting this trend. Automated CPR ensures consistent compressions during transit, improving patient outcomes. Expanding air ambulance networks in emerging economies further accelerate segment growth.
- By End User
On the basis of end user, the CPR devices market is segmented into hospital & clinic, cardiac catheterization laboratories, organ transplant units, and others. The hospital & clinic segment dominated the market in 2025, attributed to the high volume of cardiac emergencies managed within hospital settings. Hospitals maintain comprehensive emergency response systems and are early adopters of advanced CPR technologies. The presence of intensive care units, trained resuscitation teams, and reimbursement support further encourages procurement. Hospitals also require CPR devices for compliance with standardized resuscitation protocols. Continuous upgrades in emergency care infrastructure reinforce their leading position.
The cardiac catheterization laboratories segment is expected to register the fastest growth during the forecast period, driven by increasing interventional cardiology procedures and higher risk of intra-procedural cardiac arrest. CPR devices provide immediate circulatory support during complex catheter-based interventions. Rising adoption of high-risk cardiac procedures and growth in cath lab volumes are fueling demand. Automated CPR devices enable uninterrupted compressions without disrupting sterile environments. Expanding cardiac care facilities worldwide further support accelerated growth in this segment.
Cardiopulmonary Resuscitation (CRP) Devices Market Regional Analysis
- North America dominated the CPR devices market with the largest revenue share of 41.0% in 2025, supported by advanced emergency response infrastructure, high adoption of innovative resuscitation technologies, and strong presence of leading manufacturers, with the U.S. showing extensive utilization across emergency departments and coronary & intensive care units
- Healthcare providers in the region place strong emphasis on standardized, high-quality resuscitation practices, valuing CPR devices for their ability to deliver consistent chest compressions and improve survival outcomes in both in-hospital and pre-hospital settings
- This widespread adoption is further supported by advanced healthcare infrastructure, favorable reimbursement frameworks, continuous investments in emergency preparedness, and the strong presence of leading CPR device manufacturers, establishing CPR devices as essential tools across hospitals, emergency departments, and air medevac services
U.S. Cardiopulmonary Resuscitation (CRP) Devices Market Insight
The U.S. CPR devices market captured the largest revenue share of 82% in North America in 2025, fueled by the high prevalence of cardiovascular diseases and the widespread adoption of advanced life-support systems. Hospitals, emergency departments, and air medevac units are increasingly prioritizing automated CPR solutions to improve survival outcomes during sudden cardiac arrests. The growing emphasis on guideline-compliant resuscitation, coupled with strong emergency medical services infrastructure, is driving the adoption of mechanical piston devices and active compression-decompression systems. Furthermore, investments in EMS training programs and public access defibrillation initiatives are supporting the expansion of the CPR device market in the U.S.
Europe Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The Europe CPR devices market is projected to grow at a significant CAGR during the forecast period, primarily driven by increasing awareness of cardiac emergencies and stringent healthcare regulations. Hospitals and emergency medical services are adopting advanced CPR devices to ensure compliance with European Resuscitation Council guidelines. Urbanization, coupled with higher healthcare spending and focus on improving emergency care outcomes, is fostering the adoption of both mechanical and impedance threshold CPR devices. The demand is rising across emergency departments, coronary & intensive care units, and organ transplant facilities, supporting overall market expansion in Europe.
U.K. Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The U.K. CPR devices market is expected to grow at a noteworthy CAGR during the forecast period, driven by increasing cardiovascular disease incidence and the need for rapid, reliable resuscitation in hospitals and emergency units. Both public and private healthcare providers are deploying automated and mechanical CPR devices to enhance survival rates and minimize rescuer fatigue. Government initiatives promoting standardized emergency response and pre-hospital care are also contributing to growth. The integration of CPR devices in air medevac and EMT rescue units is further stimulating market demand in the U.K.
Germany Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The Germany CPR devices market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of advanced life-support technologies and investments in emergency medical infrastructure. Hospitals and cardiac care centers are adopting mechanical piston and load-distributing band devices to improve patient outcomes during sudden cardiac arrests. Germany’s focus on innovation and high-quality healthcare services promotes the integration of CPR devices in emergency departments, intensive care units, and organ transplant facilities. The growing demand for automated resuscitation solutions aligns with the country’s emphasis on precision and patient safety.
Asia-Pacific Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The Asia-Pacific CPR devices market is poised to grow at the fastest CAGR of 23% during the forecast period, driven by increasing cardiovascular disease prevalence, expanding healthcare infrastructure, and rising emergency medical services adoption in countries such as China, Japan, and India. Growing urbanization, higher disposable incomes, and government initiatives promoting emergency preparedness are boosting market growth. In addition, the increasing availability of cost-effective CPR devices and training programs for healthcare professionals are facilitating wider adoption across hospitals, air medevac units, and EMT rescue services in the region.
Japan Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The Japan CPR devices market is gaining momentum due to the country’s high focus on technological innovation and advanced healthcare systems. The aging population and rising incidence of cardiac arrest are increasing demand for mechanical and automated CPR solutions in hospitals and pre-hospital emergency services. Integration of CPR devices with hospital monitoring systems and advanced life-support protocols is supporting improved patient outcomes. Furthermore, Japan’s strong emphasis on emergency preparedness and continuous training of medical personnel is propelling the adoption of modern CPR technologies across residential and institutional healthcare settings.
India Cardiopulmonary Resuscitation (CPR) Devices Market Insight
The India CPR devices market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, growing healthcare infrastructure, and increasing cardiovascular disease prevalence. Hospitals, cardiac catheterization laboratories, and air medevac units are increasingly adopting automated and mechanical CPR devices to improve emergency response outcomes. The push towards smart hospitals, training programs for emergency medical services, and availability of cost-effective devices from domestic and international manufacturers are key factors driving market growth in India. Expanding awareness of cardiac emergencies among healthcare providers and the general population further supports the market expansion.
Cardiopulmonary Resuscitation (CRP) Devices Market Share
The Cardiopulmonary Resuscitation (CRP) Devices industry is primarily led by well-established companies, including:
- ZOLL Medical Corporation (U.S.)
- Stryker (U.S.)
- Defibtech LLC (U.S.)
- Michigan Instruments (U.S.)
- SunLife Science Inc. (U.S.)
- Schiller AG (Switzerland)
- Corpuls (Germany)
- CPR Medical Devices, Inc. (U.S.)
- Resuscitation International LLC (U.S.)
- Ambu A/S (Denmark)
- Medtronic (Ireland)
- Cardio First Angel (U.S.)
- WEINMANN Emergency Medical Technology (Germany)
- Metrax GmbH (Germany)
- Shenzhen Bangvo Technology Co., Ltd. (China)
- Ambulanc Tech Co., Ltd. (China)
- Advanced Circulatory Systems Inc. (U.S.)
- Cardiac Science Corporation (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Yantai Wanli Medical Equipment CO., Ltd. (China)
What are the Recent Developments in Global Cardiopulmonary Resuscitation (CRP) Devices Market?
- In June 2025, Mercy Air 34 (part of the Air Methods network) integrated the ZOLL AutoPulse® NXT automated CPR device into its air medical operations and achieved first responder status in central California, improving continuous CPR delivery during critical patient transports
- In June 2025, the FDA posted a Class II recall notice for Stryker’s LUCAS 2, 3, and 3.1 chest compression systems due to demonstration units being improperly provided for clinical use, requiring healthcare providers to remove affected devices and return them for appropriate replacement
- In May 2025, Air Methods, a leading U.S. air medical services provider, announced the nationwide deployment of the ZOLL AutoPulse® NXT automated CPR device across its aircraft fleet to deliver uninterrupted, high‑quality mechanical chest compressions during patient transport, enhancing both safety and outcomes for cardiac arrest patients
- In March 2025, ZOLL Circulation, Inc. issued an urgent Class I recall of its AutoPulse NXT Resuscitation System due to a failure code that could cause the device to stop compressions or deliver inadequate CPR, prompting healthcare facilities to return and repair affected units under FDA guidance
- In October 2024, a clinical observational study published in BMC Emergency Medicine highlighted the increased prevalence and impact of mechanical CPR device use in out‑of‑hospital cardiac arrest cases, analyzing how the timing of device setup correlates with patient outcomes in Taiwan
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.