Global Cardiovascular Prosthetic Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
8.86 Billion
USD
16.90 Billion
2025
2033
USD
8.86 Billion
USD
16.90 Billion
2025
2033
| 2026 –2033 | |
| USD 8.86 Billion | |
| USD 16.90 Billion | |
|
|
|
|
Cardiovascular Prosthetic Devices Market Size
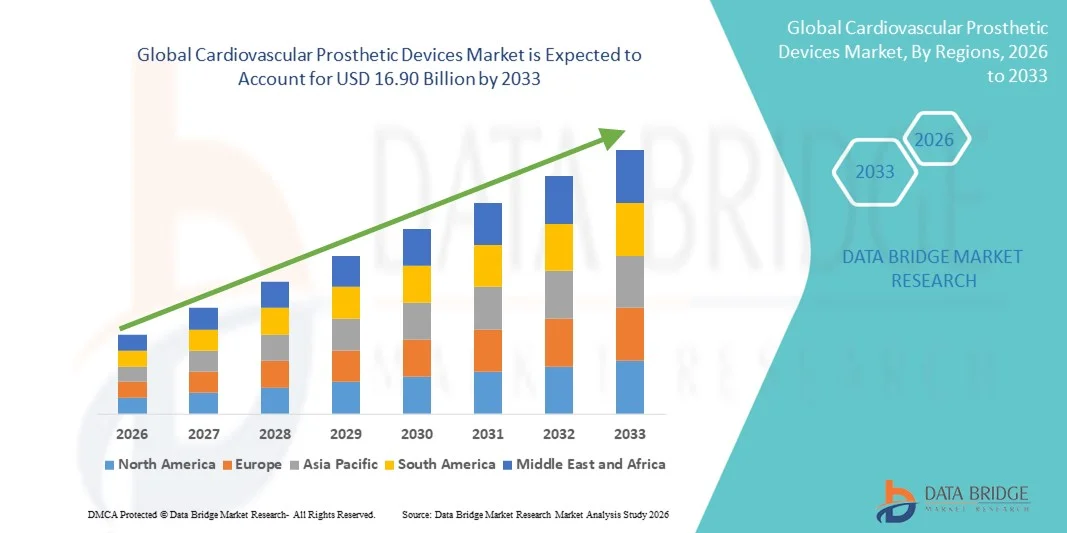
- The global cardiovascular prosthetic devices market size was valued at USD 8.86 billion in 2025 and is expected to reach USD 16.90 billion by 2033, at a CAGR of 8.41% during the forecast period
- The market growth is largely driven by the rising prevalence of cardiovascular diseases, increasing aging population, and continuous advancements in cardiovascular implant technologies, leading to greater adoption of prosthetic solutions across hospitals and cardiac care centers worldwide
- Furthermore, growing demand for minimally invasive procedures, improved patient outcomes, and long-lasting, biocompatible prosthetic devices—such as heart valves, vascular grafts, and stents—is establishing cardiovascular prosthetic devices as a critical component of modern cardiac treatment. These converging factors are accelerating the uptake of cardiovascular prosthetic device solutions, thereby significantly boosting the industry’s growth
Cardiovascular Prosthetic Devices Market Analysis
- Cardiovascular prosthetic devices, including heart valves, vascular grafts, and annuloplasty rings, are becoming essential components of modern cardiac care across hospitals and specialty cardiac centers due to their ability to restore normal cardiovascular function, improve patient survival rates, and enhance quality of life
- The increasing demand for cardiovascular prosthetic devices is primarily driven by the rising prevalence of cardiovascular diseases, a growing geriatric population, and continuous technological advancements enabling minimally invasive and transcatheter procedures with improved safety and clinical outcomes
- North America dominated the cardiovascular prosthetic devices market with the largest revenue share of 38.4% in 2025, supported by advanced healthcare infrastructure, high adoption of innovative cardiac implants, favorable reimbursement policies, and the strong presence of leading medical device manufacturers. The U.S. accounted for the majority of regional demand due to high procedure volumes and rapid uptake of next-generation prosthetic heart valves and vascular implants
- Asia-Pacific is expected to be the fastest-growing region in the cardiovascular prosthetic devices market during the forecast period, projected to expand at a CAGR of 21.2%, driven by increasing healthcare expenditure, rising awareness of cardiovascular health, expanding hospital infrastructure, and growing access to advanced cardiac surgical and interventional procedures in countries such as China and India
- The surgery segment dominated the market with the largest revenue share of 71.2% in 2025, driven by the high volume of cardiovascular surgical procedures performed worldwide
Report Scope and Cardiovascular Prosthetic Devices Market Segmentation
|
Attributes |
Cardiovascular Prosthetic Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cardiovascular Prosthetic Devices Market Trends
Advancements in Minimally Invasive and Next-Generation Cardiovascular Prosthetics
- A significant and accelerating trend in the global cardiovascular prosthetic devices market is the continuous advancement of minimally invasive technologies and next-generation prosthetic designs, including transcatheter heart valves, bioengineered grafts, and polymer-based vascular prostheses. These innovations are transforming cardiovascular interventions by reducing surgical trauma, shortening hospital stays, and improving long-term patient outcomes
- For instance, the increasing adoption of transcatheter aortic valve replacement (TAVR) prosthetic valves has significantly expanded treatment access for patients who are at high or intermediate surgical risk. Devices such as balloon-expandable and self-expanding heart valve prostheses are being widely utilized in both developed and emerging healthcare systems
- Technological advancements in material science, including the use of biocompatible polymers, tissue-engineered materials, and enhanced coatings, are improving the durability, hemocompatibility, and performance of cardiovascular prosthetic devices. These innovations help reduce complications such as thrombosis, calcification, and device degeneration over time
- The integration of advanced imaging compatibility and patient-specific prosthetic designs is further enhancing procedural precision. Customized cardiovascular prosthetics tailored to patient anatomy support better device placement and long-term functional outcomes, particularly in complex cardiac and vascular procedures
- This trend toward less invasive, more durable, and patient-centric cardiovascular prosthetic solutions is reshaping clinical practices and expanding the eligible patient population for prosthetic implantation procedures worldwide
- As a result, leading medical device manufacturers are increasingly investing in research and development to introduce innovative cardiovascular prosthetic devices that align with evolving clinical guidelines and surgeon preferences
Cardiovascular Prosthetic Devices Market Dynamics
Driver
Rising Prevalence of Cardiovascular Diseases and Aging Population
- The growing global burden of cardiovascular diseases, including coronary artery disease, valvular heart disorders, and peripheral vascular diseases, is a major driver fueling demand for cardiovascular prosthetic devices. Increasing incidence rates are directly contributing to higher volumes of surgical and interventional procedures requiring prosthetic solutions
- For instance, the rising number of patients diagnosed with aortic stenosis and heart valve dysfunction has significantly increased the adoption of prosthetic heart valves in both surgical and transcatheter procedures, particularly among elderly populations
- The global aging population is another critical factor driving market growth, as older individuals are more susceptible to cardiovascular conditions that necessitate prosthetic interventions. Age-related degeneration of heart valves and blood vessels increases the need for replacement and repair procedures
- Advancements in healthcare infrastructure, improved access to cardiovascular care, and increasing awareness regarding early diagnosis and treatment are further accelerating the utilization of cardiovascular prosthetic devices across hospitals and specialty cardiac centers
- In addition, favorable reimbursement policies in developed regions and expanding healthcare investments in emerging economies are supporting higher procedure volumes, thereby strengthening market growth prospects
Restraint/Challenge
High Device Costs and Risk of Post-Implantation Complications
- The high cost associated with advanced cardiovascular prosthetic devices remains a significant challenge for widespread adoption, particularly in cost-sensitive and low-resource healthcare settings. Premium prosthetic valves and vascular grafts can substantially increase overall treatment expenses
- For instance, transcatheter heart valve prostheses are often significantly more expensive than conventional surgical alternatives, limiting their accessibility in developing regions and smaller healthcare facilities with constrained budgets
- Despite technological advancements, the risk of post-implantation complications such as device failure, thrombosis, infection, and structural deterioration continues to raise concerns among clinicians and patients. These risks necessitate long-term monitoring and, in some cases, repeat interventions
- Strict regulatory requirements and lengthy approval processes for cardiovascular prosthetic devices can delay product commercialization and increase development costs for manufacturers, further impacting market expansion
- Addressing these challenges through cost-optimization strategies, improved clinical outcomes, enhanced device durability, and broader reimbursement coverage will be critical for sustaining long-term growth in the cardiovascular prosthetic devices market
Cardiovascular Prosthetic Devices Market Scope
The market is segmented on the basis of type, application, and end user.
- By Types
On the basis of type, the Global Cardiovascular Prosthetic Devices market is segmented into valves and pacemakers. The valves segment dominated the market with the largest revenue share of 58.4% in 2025, driven by the high prevalence of valvular heart diseases such as aortic stenosis and mitral regurgitation. Rising aging population globally has significantly increased the demand for heart valve replacement procedures. Technological advancements in bioprosthetic and mechanical valves have improved durability and patient outcomes. Increasing adoption of minimally invasive procedures such as transcatheter aortic valve replacement (TAVR) further supports segment dominance. Strong clinical evidence supporting valve efficacy drives physician preference. Favorable reimbursement policies in developed regions encourage adoption. Growing awareness of early diagnosis of valvular disorders also contributes to procedural volumes. Expansion of cardiac care centers globally continues to strengthen this segment’s leadership.
The pacemakers segment is expected to witness the fastest CAGR of 9.6% from 2026 to 2033, driven by rising incidence of arrhythmias and conduction disorders. Increasing prevalence of lifestyle-related cardiovascular diseases significantly boosts demand. Advancements in leadless pacemakers and MRI-compatible devices improve safety and adoption. Growing geriatric population is a major contributor to pacemaker implantation rates. Technological innovations enabling remote monitoring enhance patient management. Expanding access to cardiac electrophysiology services supports growth. Increased physician awareness and patient acceptance further accelerate adoption. Rising investments in cardiac device R&D strengthen future market expansion.
- By Application
On the basis of application, the Global Cardiovascular Prosthetic Devices market is segmented into surgery and research. The surgery segment dominated the market with the largest revenue share of 71.2% in 2025, driven by the high volume of cardiovascular surgical procedures performed worldwide. Rising burden of coronary artery disease, heart failure, and congenital heart defects supports surgical demand. Cardiovascular prosthetic devices are critical in valve replacement, rhythm management, and structural heart repair surgeries. Advancements in surgical techniques and device precision improve procedural success rates. Increasing availability of specialized cardiac hospitals further strengthens this segment. Strong reimbursement frameworks support surgical interventions. Growing adoption of minimally invasive and hybrid surgical approaches enhances utilization. Rising healthcare spending in emerging markets contributes to sustained dominance.
The research segment is projected to grow at the fastest CAGR of 10.8% from 2026 to 2033, driven by increasing focus on cardiovascular innovation and next-generation prosthetic development. Academic institutions and research organizations are investing heavily in device testing and validation. Growth in clinical trials for novel prosthetic materials supports demand. Collaborations between medical device companies and research institutes accelerate innovation. Increasing funding for cardiovascular research from governments and private entities boosts activity. Development of bioengineered and smart prosthetics further drives growth. Expanding translational research initiatives strengthen segment momentum. Long-term innovation pipelines support sustained expansion.
- By End User
On the basis of end user, the Global Cardiovascular Prosthetic Devices market is segmented into hospitals, clinics/cardiac centers, ambulatory surgical centers, and others. The hospitals segment dominated the market with the largest revenue share of 49.6% in 2025, driven by the availability of advanced surgical infrastructure and specialized cardiac care units. Hospitals manage complex cardiovascular procedures requiring multidisciplinary expertise. Presence of experienced cardiothoracic surgeons supports higher procedural volumes. Advanced diagnostic and imaging facilities enhance surgical precision. Hospitals benefit from strong reimbursement coverage for cardiovascular surgeries. Growing patient preference for hospital-based care supports dominance. Continuous upgrades in surgical equipment further strengthen adoption. High emergency case handling capacity reinforces leadership.
The clinics/cardiac centers segment is expected to register the fastest CAGR of 11.9% from 2026 to 2033, driven by the rapid expansion of specialized cardiac centers globally. These centers offer focused cardiovascular care with shorter patient wait times. Increasing preference for outpatient and minimally invasive procedures supports growth. Lower operational costs compared to hospitals encourage device adoption. Growing private healthcare investments accelerate center expansion. Rising medical tourism in cardiac care further boosts demand. Technological advancements allow complex procedures in specialized clinics. Improved patient convenience strengthens long-term growth prospects.
Cardiovascular Prosthetic Devices Market Regional Analysis
- North America dominated the cardiovascular prosthetic devices market with the largest revenue share of 38.4% in 2025, supported by highly advanced healthcare infrastructure, strong adoption of innovative cardiac implants, and the presence of leading medical device manufacturers
- The region benefits from high awareness of cardiovascular diseases, early diagnosis, and widespread availability of advanced surgical and interventional cardiology procedures. Favorable reimbursement policies for heart valve replacement, pacemaker implantation, and vascular prosthetic procedures further support market dominance
- Continuous technological advancements in transcatheter heart valves and minimally invasive cardiac devices are accelerating adoption. High healthcare expenditure and strong clinical expertise across hospitals and cardiac centers reinforce North America’s leadership position
U.S. Cardiovascular Prosthetic Devices Market Insight
The U.S. cardiovascular prosthetic devices market accounted for the majority of regional demand in 2025, driven by high procedure volumes and rapid uptake of next-generation prosthetic heart valves, pacemakers, and vascular implants. The country has a high prevalence of cardiovascular diseases due to aging population and lifestyle-related risk factors. Strong clinical trial activity and early adoption of innovative technologies support market growth. Favorable reimbursement frameworks encourage the use of advanced prosthetic devices in both surgical and catheter-based interventions. The presence of major manufacturers and continuous product launches further strengthen the U.S. market. Increasing focus on minimally invasive and transcatheter procedures continues to drive sustained demand.
Europe Cardiovascular Prosthetic Devices Market Insight
The Europe cardiovascular prosthetic devices market is projected to expand at a substantial CAGR during the forecast period, supported by rising incidence of cardiovascular diseases and an aging population. Well-established public healthcare systems across Western Europe enable widespread access to cardiac surgical procedures. Increasing adoption of transcatheter valve replacement and advanced vascular prosthetics is driving market growth. Strong regulatory support for medical device innovation and clinical research further accelerates adoption. Countries across the region are investing in modernizing cardiac care infrastructure. Growing emphasis on early intervention and improved patient outcomes supports sustained expansion across Europe.
U.K. Cardiovascular Prosthetic Devices Market Insight
The U.K. cardiovascular prosthetic devices market is anticipated to grow steadily during the forecast period, driven by increasing demand for cardiac surgeries and interventional cardiology procedures. The National Health Service (NHS) plays a key role in ensuring access to advanced cardiovascular treatments. Rising adoption of minimally invasive heart valve replacement and pacemaker technologies is supporting market growth. Increasing prevalence of coronary artery disease and heart rhythm disorders further fuels demand. Ongoing investments in cardiac centers of excellence and specialist training enhance procedural volumes. The U.K.’s strong clinical research environment also contributes to innovation and adoption.
Germany Cardiovascular Prosthetic Devices Market Insight
Germany cardiovascular prosthetic devices market represents one of the largest markets for cardiovascular prosthetic devices in Europe, supported by its advanced healthcare infrastructure and strong emphasis on medical innovation. The country has a high volume of cardiovascular procedures, particularly in valve replacement and vascular surgeries. Early adoption of technologically advanced prosthetic devices drives market growth. Strong reimbursement coverage and a well-developed hospital network support widespread utilization. Germany’s leadership in medical device manufacturing and clinical research further strengthens market expansion. Continuous upgrades in cardiac care facilities reinforce long-term demand.
Asia-Pacific Cardiovascular Prosthetic Devices Market Insight
The Asia-Pacific cardiovascular prosthetic devices market is expected to be the fastest-growing region during the forecast period, projected to expand at a CAGR of 21.2% from 2026 to 2033. Growth is driven by increasing healthcare expenditure, rising awareness of cardiovascular health, and expanding hospital infrastructure. Rapid urbanization and lifestyle changes are contributing to a higher prevalence of heart diseases across the region. Governments are investing heavily in improving access to advanced cardiac care. Expanding availability of skilled cardiac surgeons and interventional cardiologists further supports adoption. Growing medical tourism in cardiac procedures also boosts regional demand.
Japan Cardiovascular Prosthetic Devices Market Insight
The Japan cardiovascular prosthetic devices market is gaining steady traction due to the country’s rapidly aging population and high prevalence of cardiovascular disorders. Japan has one of the highest proportions of elderly individuals globally, significantly increasing demand for heart valve replacements and pacemaker implantations. Strong emphasis on minimally invasive and catheter-based procedures supports market growth. Advanced hospital infrastructure and high adoption of innovative medical technologies enhance procedural outcomes. Continuous investments in cardiovascular research and device innovation further strengthen the market. Favorable reimbursement policies support widespread utilization of advanced prosthetic devices.
China Cardiovascular Prosthetic Devices Market Insight
China cardiovascular prosthetic devices market accounted for the largest revenue share within the Asia-Pacific region in 2025, driven by a rapidly growing patient population and expanding access to advanced cardiac care. Rising incidence of cardiovascular diseases due to urbanization and lifestyle changes is a key growth driver. Government initiatives to modernize healthcare infrastructure and expand cardiac specialty hospitals are accelerating adoption. Increasing availability of skilled cardiac surgeons and interventional facilities supports procedural growth. Domestic manufacturers are improving affordability and accessibility of cardiovascular prosthetic devices. Growing awareness of early diagnosis and treatment further strengthens market expansion in China.
Cardiovascular Prosthetic Devices Market Share
The Cardiovascular Prosthetic Devices industry is primarily led by well-established companies, including:
• Medtronic (Ireland )
• Abbott (U.S.)
• Boston Scientific (U.S.)
• Edwards Lifesciences (U.S.)
• Johnson & Johnson (U.S.)
• Biotronik (Germany)
• LivaNova (U.K.)
• Terumo Corporation (Japan)
• Stryker Corporation (U.S.)
• MicroPort Scientific Corporation (China)
• Merit Medical Systems (U.S.)
• B. Braun Melsungen AG (Germany)
• Cook Medical (U.S.)
Latest Developments in Global Cardiovascular Prosthetic Devices Market
- In March 2021, Medtronic plc received U.S. FDA approval for its Harmony™ Transcatheter Pulmonary Valve (TPV), the first minimally invasive therapy for patients with severe pulmonary regurgitation associated with congenital heart disease, enabling a catheter-based approach that reduces the need for open-heart surgery in both adult and pediatric patients
- In August 2021, Medtronic announced FDA approval of its next-generation Evolut FX Transcatheter Aortic Valve Replacement (TAVR) system, which enhances ease-of-use and procedural precision for treatment of symptomatic severe aortic stenosis, reinforcing its leadership in transcatheter heart valve technology
- In August 2023, Genesis MedTech’s J-Valve Transfemoral System was granted Breakthrough Device designation by the U.S. FDA for the treatment of severe native aortic regurgitation and mixed aortic valve disease via a minimally invasive approach, expediting development and regulatory communication for this novel prosthetic valve system
- In March 2024, Medtronic announced FDA approval of the newest-generation Evolut FX+ TAVR system for the treatment of symptomatic severe aortic stenosis, featuring design enhancements that facilitate coronary access without compromising valve performance, representing an important advancement in TAVR technology
- In February 2024, Edwards Lifesciences’ EVOQUE™ Tricuspid Valve replacement system became the first transcatheter therapy approved by the U.S. FDA for the treatment of tricuspid regurgitation, offering a minimally invasive option for patients who have not responded adequately to optimal medical therapy
- In May 2025, Edwards Lifesciences announced that its SAPIEN 3 Transcatheter Aortic Valve Replacement (TAVR) platform received FDA approval for use in patients with severe aortic stenosis who are asymptomatic, expanding the clinical patient population eligible for minimally invasive valve replacement
- In January 2025, Abbott Laboratories reported successful completion of the EVOLUT PRO+ Transcatheter Valve Replacement clinical trial, demonstrating improved procedural efficiency for patients with smaller or more complex aortic annuli and expanding the potential applicability of TAVR therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.