Global Celiac Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
1.42 Billion
USD
3.09 Billion
2024
2032
USD
1.42 Billion
USD
3.09 Billion
2024
2032
| 2025 –2032 | |
| USD 1.42 Billion | |
| USD 3.09 Billion | |
|
|
|
|
Celiac Drugs Market Size
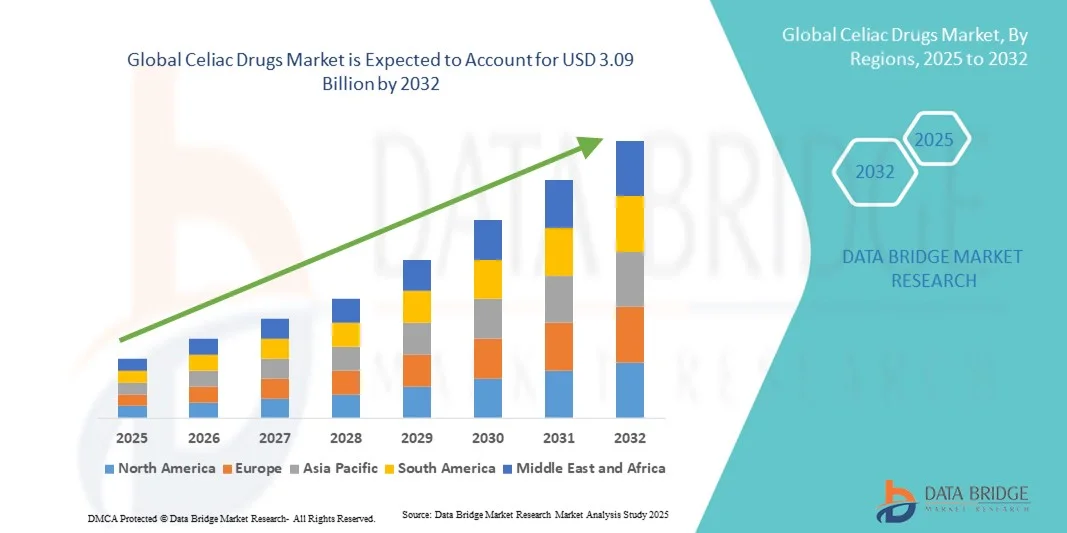
- The global celiac drugs market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 3.09 billion by 2032, at a CAGR of 10.19% during the forecast period
- The market growth is largely fueled by the increasing prevalence of celiac disease worldwide, ongoing advancements in diagnostic technologies, and the development of novel therapeutic options, driving adoption of effective treatments
- Furthermore, rising consumer demand for targeted, safe, and convenient drug therapies, along with the expansion of gluten-free product offerings, is establishing pharmaceutical interventions as the preferred management option for patients. These converging factors are accelerating the uptake of celiac drugs, thereby significantly boosting the industry's growth
Celiac Drugs Market Analysis
- Celiac drugs, offering pharmaceutical interventions for managing celiac disease, are increasingly vital components of patient care in both adult and pediatric populations due to their targeted efficacy, improved safety profiles, and integration with dietary management plans
- The escalating demand for celiac drugs is primarily fueled by the rising prevalence of celiac disease globally, growing awareness among patients and healthcare providers, and a rising preference for effective, non-dietary treatment options
- North America dominated the celiac drugs market with the largest revenue share of 35.4% in 2024, characterized by advanced healthcare infrastructure, high patient awareness, and a strong presence of key pharmaceutical companies, with the U.S. experiencing substantial growth in drug adoption, particularly in hospitals and specialty clinics, driven by innovations from both established pharma companies and biotech startups
- Asia-Pacific is expected to be the fastest growing region in the celiac drugs market during the forecast period due to increasing disease awareness, improving healthcare access, and rising disposable incomes
- Biological drugs segment dominated the celiac drugs market with a market share of 42.2% in 2024, driven by their established efficacy in managing immune responses associated with celiac disease and increasing adoption in classical and non-classical celiac disease cases
Report Scope and Celiac Drugs Market Segmentation
|
Attributes |
Celiac Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Celiac Drugs Market Trends
Advancements in Targeted and Biological Therapies
- A significant and accelerating trend in the global celiac drugs market is the growing development of targeted biological therapies and enzyme-based treatments, which aim to improve treatment efficacy and reduce side effects for patients with celiac disease
- For instance, emerging enzyme therapy products are designed to degrade gluten in the gastrointestinal tract, reducing immune response and improving patient quality of life. Similarly, novel biologics such as monoclonal antibodies target specific immune pathways to manage inflammation and intestinal damage
- Targeted therapies enable personalized treatment approaches, allowing clinicians to select drugs based on disease subtype (classical, non-classical, or silent celiac disease) and patient response, enhancing therapeutic outcomes and minimizing adverse effects
- The integration of innovative drug modalities with dietary management and patient monitoring systems facilitates more comprehensive celiac disease care. Through such integrated approaches, patients can manage symptoms more effectively while improving adherence to gluten-free diets
- This trend towards biologically precise, patient-centric, and integrated treatment regimens is fundamentally reshaping expectations for celiac disease management. Consequently, companies such as ImmusanT and Nexvax2 are developing therapies that target specific immune mechanisms while complementing dietary therapy
- The demand for celiac drugs with targeted, safe, and effective mechanisms of action is growing rapidly across both adult and pediatric populations, as healthcare providers increasingly prioritize therapeutic efficacy and improved patient quality of life
Celiac Drugs Market Dynamics
Driver
Increasing Prevalence and Rising Awareness of Celiac Disease
- The growing prevalence of celiac disease worldwide, coupled with rising awareness among patients and healthcare providers, is a significant driver for the heightened demand for celiac drugs
- For instance, in 2024, new patient screening programs in North America and Europe have led to earlier diagnosis and greater adoption of pharmaceutical interventions, driving market growth
- As more patients are diagnosed with classical and non-classical celiac disease, the demand for effective pharmacological treatments alongside gluten-free diets increases, providing improved symptom management
- Furthermore, increasing educational initiatives by celiac associations and advocacy groups are improving patient knowledge and compliance, which is boosting drug uptake
- The convenience of oral, parenteral, and topical drug formulations, coupled with enhanced accessibility through hospitals, specialty clinics, and online pharmacies, is propelling adoption in both developed and emerging markets
- The trend towards personalized medicine and combination therapies, alongside expanding insurance coverage, is further driving the uptake of celiac drugs across different patient demographics
Restraint/Challenge
Regulatory Hurdles and Limited Awareness in Emerging Markets
- Strict regulatory requirements for clinical trials and drug approvals pose a significant challenge to new entrants and limit the speed of new celiac drug launches
- For instance, prolonged approval timelines and rigorous efficacy and safety testing can delay market entry for novel therapies, restricting early adoption
- Limited awareness of celiac disease in emerging markets, combined with underdiagnosis, reduces demand for pharmaceutical interventions, affecting market expansion potential
- In addition, high costs of biologics and advanced therapies compared to dietary management options can be a barrier for price-sensitive patients, particularly in low- and middle-income countries
- While awareness and accessibility are gradually improving through government and NGO programs, the perceived complexity of medical therapies versus gluten-free diets may hinder widespread acceptance
- Overcoming these challenges through accelerated regulatory pathways, increased patient education, and development of cost-effective therapies will be crucial for sustained market growth
Celiac Drugs Market Scope
The market is segmented on the basis of type, application, route of administration, end-users, and distribution channel.
- By Type
On the basis of type, the celiac drugs market is segmented into biological drugs, immunosuppressants, corticosteroids, and others. The biological drugs segment dominated the market with the largest market revenue share of 42.2% in 2024, driven by their high efficacy in targeting immune-mediated damage associated with celiac disease. Patients and healthcare providers often prefer biological drugs for severe and refractory cases due to their precision and ability to reduce intestinal inflammation effectively. The market also sees strong demand for biological drugs due to ongoing innovation, such as monoclonal antibodies and enzyme therapies, which complement gluten-free dietary interventions. Clinical studies continue to demonstrate improved patient outcomes, reinforcing their leading position. In addition, biologics are often used in combination with standard therapies, ensuring comprehensive management of symptoms and complications. The growing pipeline of biologic therapies under development further strengthens dominance in this segment.
The immunosuppressants segment is anticipated to witness the fastest growth rate of 12.4% from 2025 to 2032, fueled by their growing adoption in non-classical and refractory celiac disease cases. Immunosuppressants help manage overactive immune responses and provide an alternative for patients who do not respond adequately to dietary management or biological therapies. The increasing awareness among clinicians about the benefits of targeted immunosuppressive therapy is also contributing to rapid adoption. Improvements in safety profiles and personalized treatment approaches are attracting more patients toward this segment. Immunosuppressants are also being integrated with emerging combination therapies, further enhancing growth prospects. In addition, their expanding presence in specialty clinics and hospitals supports accelerated uptake across global markets.
- By Application
On the basis of application, the celiac drugs market is segmented into classical celiac disease, non-classical celiac disease, and silent celiac disease. The classical celiac disease segment dominated the market in 2024 due to the high prevalence of patients exhibiting typical gastrointestinal symptoms, which necessitate pharmacological intervention alongside gluten-free diets. Healthcare providers prioritize treatment in classical cases to prevent severe malabsorption and associated complications. The availability of approved drugs and clinical guidelines ensures steady adoption. Ongoing patient education and awareness programs further reinforce market dominance. Pharmaceutical companies continue to focus on enhancing efficacy, reducing side effects, and improving adherence. In addition, hospital-based treatment and monitoring support wide adoption in this segment.
The non-classical celiac disease segment is expected to witness the fastest growth from 2025 to 2032, driven by rising diagnosis rates of atypical and extra-intestinal manifestations. Improved diagnostic methods, including serology and genetic testing, are expanding the patient pool. Clinicians are increasingly adopting targeted therapies for non-classical presentations. Patient preference for early intervention and improved quality of life is further driving uptake. The development of new biologics and immunosuppressants tailored for non-classical disease enhances growth. Awareness campaigns by healthcare organizations are also contributing to faster adoption.
- By Route of Administration
On the basis of route of administration, the celiac drugs market is segmented into topical, parenteral, oral, and others. The oral segment dominated the market in 2024 due to patient preference for non-invasive administration and ease of long-term adherence. Oral medications, including enzyme therapies and corticosteroids, are convenient for daily use and compatible with dietary management plans. Clinicians favor oral administration to improve compliance and minimize hospital visits. The wide availability of oral formulations in retail and hospital pharmacies supports dominance. Pharmaceutical innovations to improve bioavailability and reduce gastrointestinal side effects further strengthen this segment. Patients also benefit from the flexibility of self-administration, enhancing overall treatment adherence.
The parenteral segment is expected to witness the fastest growth rate from 2025 to 2032, driven by increased adoption of injectable biological drugs for severe or refractory cases. Parenteral administration ensures rapid systemic delivery and effective immune modulation. Hospitals and specialty clinics prefer parenteral drugs due to controlled administration and predictable pharmacokinetics. Emerging biologics and monoclonal antibodies are primarily available in injectable form, fueling growth. Rising awareness among clinicians regarding parenteral therapies enhances adoption. In addition, patient education and support programs are helping increase acceptance of injectable treatments.
- By End-Users
On the basis of end-users, the celiac drugs market is segmented into hospitals, specialty clinics, home healthcare, and others. The hospitals segment dominated the market in 2024, accounting for the largest revenue share due to the high volume of diagnosed celiac patients requiring supervision during treatment initiation and monitoring. Hospitals provide access to advanced therapies, diagnostic support, and multidisciplinary care, making them the preferred treatment setting. Government and private hospital programs often facilitate reimbursement, improving patient access to high-cost drugs. Hospitals also serve as centers for clinical trials, supporting awareness and adoption of new therapies. Hospital pharmacies ensure proper storage, dispensing, and monitoring of biological and immunosuppressive drugs. The strong presence of hospitals in developed regions reinforces the segment’s dominance.
The specialty clinics segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing establishment of gastroenterology and celiac-focused clinics. These clinics offer targeted care, patient education, and ongoing monitoring, enhancing therapy adherence and outcomes. Specialty clinics are increasingly integrating advanced biologics and enzyme therapies into treatment protocols. Rising patient preference for personalized care and convenience further supports growth. Expansion of specialty clinics in emerging markets is contributing to higher adoption. Partnerships with pharmaceutical companies for patient support programs accelerate uptake in this segment.
- By Distribution Channel
On the basis of distribution channel, the celiac drugs market is segmented into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment dominated the market in 2024 due to direct access to in-patient and out-patient patients requiring specialized therapies, particularly biological and injectable drugs. Hospital pharmacies ensure proper storage, dispensing, and monitoring, making them a trusted source for patients and clinicians. The strong hospital network in developed regions supports consistent supply and adoption. Integration with insurance and reimbursement systems enhances affordability. Hospitals also educate patients on proper administration and adherence, reinforcing trust. The collaboration of hospital pharmacies with clinical trials accelerates awareness and acceptance of new drugs.
The online pharmacy segment is expected to witness the fastest growth rate from 2025 to 2032, driven by increasing digitalization, patient preference for home delivery, and growing awareness of e-pharmacy platforms. Online pharmacies provide convenient access to oral medications and supportive supplements, especially in regions with limited physical pharmacy infrastructure. Integration with telemedicine platforms further enhances adoption. Regulatory support for e-pharmacies and rising trust in digital healthcare contribute to growth. Patient education on safe online procurement is increasing acceptance. Partnerships with pharmaceutical companies ensure steady supply and brand trust, driving rapid growth.
Celiac Drugs Market Regional Analysis
- North America dominated the celiac drugs market with the largest revenue share of 35.4% in 2024, characterized by advanced healthcare infrastructure, high patient awareness, and a strong presence of key pharmaceutical companies
- Patients and clinicians in the region highly value the availability of advanced therapies, including biological drugs, immunosuppressants, and enzyme-based treatments, which complement gluten-free dietary management plans
- This widespread adoption is further supported by strong hospital and specialty clinic networks, well-established insurance coverage, and government programs promoting early diagnosis and treatment, establishing celiac drugs as the preferred management solution for both adult and pediatric patients
U.S. Celiac Drugs Market Insight
The U.S. celiac drugs market captured the largest revenue share of 78% in 2024 within North America, fueled by the high prevalence of diagnosed celiac disease and advanced healthcare infrastructure. Patients are increasingly prioritizing effective management through biological drugs, immunosuppressants, and enzyme therapies alongside gluten-free diets. The growing adoption of specialty clinics, home healthcare, and hospital pharmacies further propels the market. Moreover, the availability of insurance coverage and government programs supporting early diagnosis and treatment is significantly contributing to market expansion. Patient awareness campaigns and educational initiatives by celiac associations are also driving uptake across both adult and pediatric populations.
Europe Celiac Drugs Market Insight
The Europe celiac drugs market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by the increasing prevalence of celiac disease and rising awareness among healthcare providers and patients. The increase in urbanization, coupled with improved healthcare infrastructure, is fostering the adoption of pharmacological interventions. European patients are drawn to the efficacy and convenience offered by advanced therapies such as biologics and enzyme treatments. The region is experiencing significant growth across hospitals, specialty clinics, and home healthcare, with celiac drugs being incorporated into both newly diagnosed cases and ongoing management programs. In addition, strong clinical guidelines and reimbursement support are promoting market expansion.
U.K. Celiac Drugs Market Insight
The U.K. celiac drugs market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising diagnosis rates, awareness of treatment options, and patient preference for effective drug therapies. Concerns regarding malnutrition, intestinal damage, and long-term complications are encouraging both clinicians and patients to adopt pharmaceutical solutions alongside dietary management. The U.K.’s well-developed healthcare and e-pharmacy infrastructure is expected to continue supporting market growth. Moreover, the increasing integration of telemedicine and online pharmacies is enhancing accessibility and adherence. Ongoing educational initiatives and support programs by patient advocacy groups are also boosting uptake in both adult and pediatric populations.
Germany Celiac Drugs Market Insight
The Germany celiac drugs market is expected to expand at a considerable CAGR during the forecast period, fueled by growing awareness of celiac disease and demand for technologically advanced, evidence-based treatments. Germany’s strong healthcare system, combined with clinical research and innovation in biological and enzyme therapies, promotes adoption in hospitals and specialty clinics. Patients are increasingly seeking safe, effective, and convenient drug therapies in addition to gluten-free dietary management. Integration of pharmacological therapies with patient monitoring systems is also becoming prevalent. Strong reimbursement support and government initiatives for early diagnosis and management further drive market growth.
Asia-Pacific Celiac Drugs Market Insight
The Asia-Pacific celiac drugs market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by rising disease awareness, improving healthcare infrastructure, and increasing disposable incomes in countries such as China, Japan, and India. The region’s growing inclination towards early diagnosis and effective drug therapies is driving adoption. Furthermore, government initiatives to improve healthcare access and the expansion of specialty clinics and hospitals are increasing the availability of advanced treatments. The affordability of oral and injectable therapies, coupled with patient education programs, is expanding the market to a wider population. Rapid urbanization and digitalization, including telemedicine and e-pharmacies, are further supporting market growth in APAC.
Japan Celiac Drugs Market Insight
The Japan celiac drugs market is gaining momentum due to high disease awareness, well-established healthcare infrastructure, and patient preference for advanced drug therapies. Japanese patients place a significant emphasis on effective management of classical and non-classical celiac disease, with adoption driven by biologics, enzyme therapies, and immunosuppressants. The integration of pharmacological treatments with hospital and specialty clinic support is fueling growth. Moreover, the aging population is such asly to spur demand for safe, easy-to-administer therapies in both adult and pediatric populations. Strong reimbursement policies and increasing patient education are further accelerating uptake in residential and clinical settings.
India Celiac Drugs Market Insight
The India celiac drugs market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to rising disease awareness, rapid urbanization, and expanding healthcare infrastructure. India is witnessing increasing adoption of oral and parenteral therapies in hospitals, specialty clinics, and home healthcare settings. The growing middle-class population, coupled with government programs promoting early diagnosis, is a key factor propelling market growth. In addition, the availability of affordable drug therapies, supported by domestic pharmaceutical manufacturers, is enhancing accessibility. Patient education initiatives and telemedicine integration further boost adoption, particularly in urban and semi-urban regions.
Celiac Drugs Market Share
The celiac drugs industry is primarily led by well-established companies, including:
- Anokion. (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Topas Therapeutics (Germany)
- ZymagenX, Inc. (U.S.)
- ZEDIRA GmbH, (U.S.)
- Amlitelimab (U.S.)
- Inverse Vaccine (U.S.)
- Merck & Co., Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- AstraZeneca (U.K.)
- AbbVie Inc. (U.S.)
- Johnson & Johnson and its affiliates (U.S.)
- Pfizer Inc. (U.S.)
- Lilly USA, LLC. (U.S.)
- Sanofi (France)
- Novartis AG (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- Regeneron Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Celiac Drugs Market?
- In August 2025, the ASPIRION study, a Phase 2 clinical trial, commenced to evaluate Amlitelimab, a monoclonal antibody targeting the immune response in celiac disease. This trial focuses on patients with non-responsive celiac disease, a condition where individuals continue to experience symptoms despite strict adherence to a gluten-free diet. The study aims to assess whether Amlitelimab can reduce intestinal damage and alleviate symptoms in these patients
- In May 2025, Teva Pharmaceutical Industries announced that the U.S. Food and Drug Administration (FDA) granted Fast Track designation to its investigational drug TEV-53408, an anti-IL-15 monoclonal antibody. This drug is currently undergoing a Phase 2a clinical trial to assess its efficacy and safety in adults with celiac disease adhering to a gluten-free diet. The Fast Track status aims to expedite the development and review process for drugs that address unmet medical needs
- In January 2025, Anokion announced positive symptom data from its Phase 2 trial evaluating KAN-101 for the treatment of celiac disease. The data indicated that KAN-101 reduced multiple gluten-induced symptoms and celiac-specific composite measures. KAN-101 was found to be safe and well-tolerated in individuals with celiac disease, marking a promising step forward in the development of treatments beyond the gluten-free diet
- In July 2024, researchers from Tampere University in Finland reported significant progress with ZED1227, a new drug designed to aid in the management of celiac disease. ZED1227 targets the enzyme transglutaminase 2 (TG2), which plays a crucial role in the harmful immune response to gluten in individuals with celiac disease. The drug is intended to be used alongside a gluten-free diet to help reduce inflammation and intestinal damage caused by accidental gluten exposure
- In June 2024, Entero Therapeutics announced the publication of a peer-reviewed paper detailing a novel algorithm designed to measure intestinal damage in individuals with celiac disease. This innovative tool represents a significant advancement in the accurate assessment and management of celiac disease, potentially leading to improved patient outcomes. The algorithm offers a more precise method for evaluating the extent of intestinal damage, which is crucial for monitoring disease progression and treatment efficacy
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.