Global Cell Based Immunotherapy Market
Market Size in USD Billion
CAGR :
% 
 USD
5.74 Billion
USD
23.09 Billion
2025
2033
USD
5.74 Billion
USD
23.09 Billion
2025
2033
| 2026 –2033 | |
| USD 5.74 Billion | |
| USD 23.09 Billion | |
|
|
|
|
Cell-Based Immunotherapy Market Size
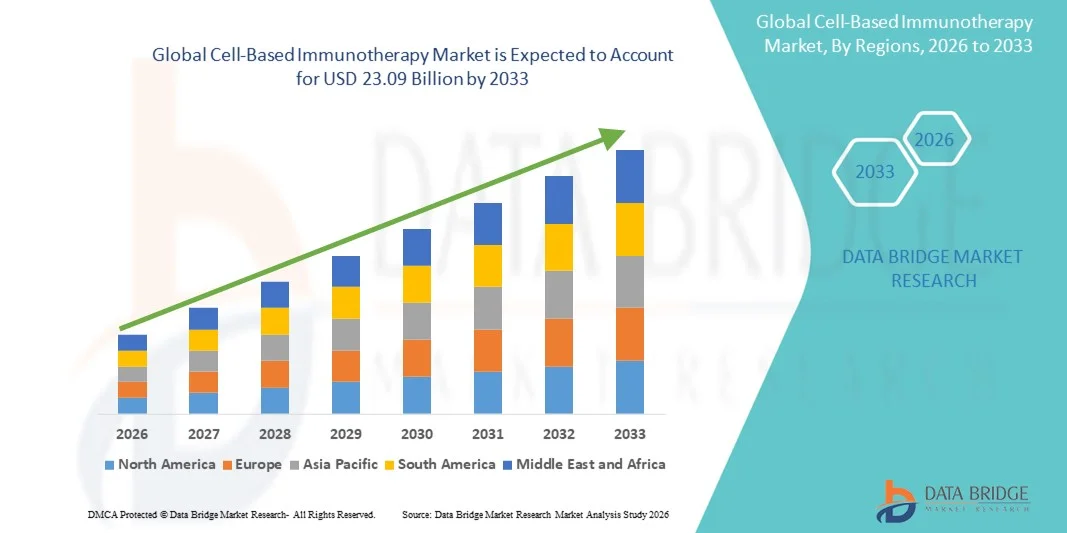
- The global cell-based immunotherapy market size was valued at USD 5.74 billion in 2025 and is expected to reach USD 23.09 billion by 2033, at a CAGR of 19.01% during the forecast period
- The market growth is largely driven by increasing incidence of cancer and chronic diseases, rapid advancements in cell engineering technologies, and growing adoption of personalized medicine approaches, enabling highly targeted and effective therapies
- Furthermore, rising investments in research and development, coupled with favorable regulatory initiatives and expanding clinical trial activities, are positioning cell-based immunotherapies as a transformative treatment option. These factors are accelerating the adoption of advanced immunotherapy solutions, thereby significantly boosting the industry's growth
Cell-Based Immunotherapy Market Analysis
- Cell-based immunotherapies, including CAR-T, TCR-T, and NK cell therapies, are increasingly recognized as transformative treatment options for cancer and other chronic diseases, offering personalized, targeted, and highly effective therapeutic interventions
- The escalating demand for cell-based immunotherapies is primarily fueled by rising incidence of cancers, growing awareness of personalized medicine, and advancements in cell engineering technologies that improve safety and efficacy
- North America dominated the cell-based immunotherapy market with the largest revenue share of 43.4% in 2025, characterized by a robust biotechnology ecosystem, high healthcare expenditure, and a strong presence of leading pharmaceutical and biotech companies, with the U.S. driving substantial growth through ongoing clinical trials, regulatory support, and innovations in cellular therapy platforms
- Asia-Pacific is expected to be the fastest growing region in the cell-based immunotherapy market during the forecast period due to increasing healthcare infrastructure investments, rising patient awareness, and supportive government initiatives
- Prostate cancer segment dominated the cell-based immunotherapy market with a market share of 31.9% in 2025, driven by increasing prevalence, targeted therapy adoption, and ongoing clinical trials demonstrating improved patient outcomes
Report Scope and Cell-Based Immunotherapy Market Segmentation
|
Attributes |
Cell-Based Immunotherapy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cell-Based Immunotherapy Market Trends
Advancements in Personalized and Targeted Therapies
- A significant and accelerating trend in the global cell-based immunotherapy market is the growing focus on personalized and highly targeted treatment approaches, enabling therapies tailored to individual patient tumor profiles and immune responses
- For instance, CAR-T therapies such as Kymriah and Yescarta are engineered to target specific antigens expressed on cancer cells, offering precise and effective treatment with minimized off-target effects
- Integration of genomic profiling and AI-driven predictive models allows clinicians to optimize therapy selection and dosing, potentially improving patient outcomes and reducing adverse effects
- The combination of cell-based immunotherapies with checkpoint inhibitors or other immune modulators is creating multi-modal treatment regimens that enhance efficacy and durability of response in challenging cancers
- Companies are increasingly focusing on allogeneic or “off-the-shelf” cell therapies to reduce treatment timelines and costs while broadening patient access
- Development of next-generation delivery platforms, such as nanoparticle-assisted or scaffold-based cell therapies, is enhancing cell survival and targeting efficiency in solid tumors
- This trend towards highly individualized, data-driven, and combinatorial therapies is reshaping patient expectations and clinical approaches, prompting companies such as Gilead and Allogene to develop next-generation cellular therapies with enhanced targeting and safety profiles
- The demand for personalized and combinatorial cell-based therapies is growing rapidly across oncology and rare disease indications, as patients and clinicians increasingly seek treatments that offer higher efficacy and lower systemic toxicity
Cell-Based Immunotherapy Market Dynamics
Driver
Rising Cancer Incidence and Demand for Advanced Therapies
- The increasing prevalence of cancers worldwide, coupled with the need for innovative and effective treatment options, is a significant driver for the adoption of cell-based immunotherapies
- For instance, in March 2025, Bristol Myers Squibb announced expansion of its clinical trials for CAR-T therapies targeting multiple hematologic malignancies, reflecting industry efforts to meet growing patient demand
- As patients seek better survival outcomes and fewer side effects than conventional therapies, cell-based immunotherapies provide highly targeted treatment modalities that can improve quality of life
- Furthermore, the growing adoption of personalized medicine and genomic profiling is facilitating the identification of suitable patients for these therapies, boosting their clinical uptake
- Increasing investments from both public and private sectors in research and development are accelerating innovation and pipeline expansion in the market
- Strategic collaborations and licensing agreements between biotech companies and academic institutions are enabling faster commercialization and global reach of novel therapies
- Strong R&D pipelines, government initiatives supporting advanced therapies, and increasing availability in specialized cancer centers are further propelling the market growth globally
Restraint/Challenge
High Cost and Complex Manufacturing Challenges
- The high cost of cell-based immunotherapies and the complexity of manufacturing personalized treatments pose significant challenges to widespread market adoption
- For instance, individualized CAR-T therapies require patient-specific cell extraction, engineering, and reinfusion, leading to long production times and high treatment costs
- Limited manufacturing capacity and logistical constraints can delay treatment delivery, impacting patient access and treatment outcomes
- Regulatory hurdles and the need for stringent quality control measures further complicate commercialization and global rollout of these therapies
- Stringent cold-chain requirements for transporting live cells increase operational complexity and costs
- Variability in patient response and potential adverse effects, such as cytokine release syndrome, necessitate careful monitoring and can limit broader acceptance
- Addressing cost, scaling manufacturing, and streamlining regulatory pathways are critical for improving accessibility and ensuring sustainable growth in the cell-based immunotherapy market
Cell-Based Immunotherapy Market Scope
The market is segmented on the basis of application and end user.
- By Application
On the basis of application, the cell-based immunotherapy market is segmented into prostate cancer, breast cancer, skin cancer, ovarian cancer, brain tumor, lung cancer, and other cancers. The prostate cancer segment dominated the market with the largest revenue share of 31.9% in 2025, driven by the high prevalence of prostate cancer in aging male populations and the growing adoption of targeted CAR-T and TCR-T therapies. Increasing awareness about advanced treatment options, coupled with favorable clinical outcomes from ongoing trials, is further bolstering market dominance. Hospitals and specialized cancer centers are increasingly adopting cell-based therapies for prostate cancer due to well-established treatment protocols. In addition, ongoing research focusing on minimizing side effects and improving patient response is strengthening the segment’s position in the market. Personalized immunotherapy solutions targeting prostate-specific antigens continue to attract significant investment, reinforcing its leadership in terms of revenue.
The brain tumor segment is anticipated to witness the fastest growth rate of 23.6% from 2026 to 2033, fueled by increasing unmet medical needs in glioblastoma and other aggressive brain cancers. Advancements in gene editing, CAR-T therapy modifications, and immune cell penetration across the blood-brain barrier are driving the rapid adoption of therapies targeting brain tumors. Rising clinical trial activity and collaborations between biotech companies and research institutions are accelerating innovation in this space. The growing patient population and limited effectiveness of conventional treatments further push demand for innovative immunotherapies. Moreover, government and private funding initiatives for rare and difficult-to-treat cancers are expected to support segment growth. Emerging therapies offering personalized targeting and improved safety profiles are making brain tumor applications a high-growth opportunity.
- By End User
On the basis of end user, the market is segmented into hospitals, ambulatory surgical centers, and specialized cancer institutes. The hospitals segment dominated the market in 2025, owing to the availability of advanced infrastructure, specialized oncology departments, and high patient throughput capable of supporting complex cell-based therapy administration. Hospitals provide comprehensive care, including pre-treatment screening, therapy administration, and post-treatment monitoring, making them the preferred choice for most patients. Furthermore, hospitals often have access to the latest technology and experienced medical staff, which supports adoption of high-cost and advanced therapies. Strategic partnerships with biotech companies for clinical trials and expanded treatment offerings also contribute to dominance. The segment benefits from both established and newly approved therapies being primarily delivered through hospital settings.
The specialized cancer institutes segment is expected to witness the fastest growth from 2026 to 2033, driven by their focus on high-complexity treatments and research-oriented patient care. These institutes offer personalized treatment plans, advanced diagnostic facilities, and access to clinical trials, making them ideal for innovative immunotherapies. Growth is supported by increasing patient preference for specialized care and targeted therapy outcomes. Institutes also benefit from collaborations with pharmaceutical and biotechnology companies to implement cutting-edge therapies rapidly. The ability to manage rare, complex, or multi-modality cancer treatments enhances their appeal as a high-growth end-user segment. In addition, investment in training and specialized personnel ensures efficient administration of next-generation cell-based therapies.
Cell-Based Immunotherapy Market Regional Analysis
- North America dominated the cell-based immunotherapy market with the largest revenue share of 43.4% in 2025, characterized by a robust biotechnology ecosystem, high healthcare expenditure, and a strong presence of leading pharmaceutical and biotech companies
- Patients and healthcare providers in the region highly value the advanced efficacy, targeted treatment approaches, and personalized therapy options offered by cell-based immunotherapies, particularly for hematologic malignancies and other complex cancers
- This widespread adoption is further supported by favorable regulatory frameworks, robust clinical trial activity, and significant investment in research and development, establishing cell-based immunotherapies as a preferred treatment option in both hospitals and specialized cancer institutes across North America
U.S. Cell-Based Immunotherapy Market Insight
The U.S. cell-based immunotherapy market captured the largest revenue share of 80% in 2025 within North America, fueled by a robust biotechnology ecosystem and widespread adoption of advanced cancer treatments. Patients and healthcare providers are increasingly prioritizing personalized, targeted therapies for hematologic malignancies and other complex cancers. The growing number of clinical trials, combined with strong government and private funding for immunotherapy research, further propels market growth. Moreover, the presence of leading pharmaceutical and biotech companies driving innovation and commercialization is significantly contributing to the market's expansion.
Europe Cell-Based Immunotherapy Market Insight
The Europe cell-based immunotherapy market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing prevalence of cancers and supportive healthcare infrastructure. The rising awareness of advanced therapies and government initiatives promoting access to novel treatments are fostering adoption. European patients are also drawn to personalized and targeted immunotherapies, which offer improved outcomes and reduced side effects. The region is witnessing significant growth across hospitals and specialized cancer institutes, with cell-based therapies increasingly incorporated into treatment protocols for both new and relapsed cancer cases.
U.K. Cell-Based Immunotherapy Market Insight
The U.K. cell-based immunotherapy market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the adoption of precision medicine and increasing investment in advanced cancer therapies. Concerns regarding limited efficacy of conventional treatments are encouraging both hospitals and specialized institutes to adopt cell-based therapies. The U.K.’s strong healthcare infrastructure, combined with clinical trial activities and collaborations with biotech firms, is expected to continue stimulating market growth.
Germany Cell-Based Immunotherapy Market Insight
The Germany cell-based immunotherapy market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s strong focus on innovation, research, and advanced healthcare solutions. Germany’s well-developed hospital and specialized cancer institute network supports the administration of complex therapies. The integration of cell-based therapies into clinical practice, along with government support for innovative treatments, is driving adoption. Patients and clinicians increasingly prefer advanced, personalized options for cancer care, aligning with local expectations for efficacy and safety.
Asia-Pacific Cell-Based Immunotherapy Market Insight
The Asia-Pacific cell-based immunotherapy market is poised to grow at the fastest CAGR of 25% during the forecast period of 2026 to 2033, driven by increasing cancer prevalence, rising healthcare investments, and expanding access to advanced therapies in countries such as China, Japan, and India. The region’s growing focus on healthcare innovation and supportive government initiatives is fueling adoption. Furthermore, as APAC emerges as a hub for biotechnology research and clinical trials, accessibility and affordability of cell-based immunotherapies are expanding to a wider patient population.
Japan Cell-Based Immunotherapy Market Insight
The Japan cell-based immunotherapy market is gaining momentum due to the country’s aging population, high healthcare standards, and demand for innovative cancer treatments. The Japanese market places significant emphasis on efficacy and safety, and adoption is driven by increasing clinical trial activity and integration of personalized therapies into hospitals and specialized institutes. Moreover, Japan’s advanced healthcare infrastructure supports complex therapy administration, ensuring wider access for patients requiring high-precision treatments.
India Cell-Based Immunotherapy Market Insight
The India cell-based immunotherapy market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to the country’s growing patient population, rising cancer incidence, and expanding healthcare infrastructure. India is witnessing increasing adoption of advanced therapies in hospitals and specialized cancer institutes. The push towards affordable and accessible innovative treatments, coupled with collaborations with global biotech firms, is propelling the market. Strong government initiatives to improve cancer care and clinical research further support growth in the Indian market.
Cell-Based Immunotherapy Market Share
The Cell-Based Immunotherapy industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Adaptimmune (U.S.)
- Fate Therapeutics, Inc. (U.S.)
- Allogene Therapeutics (U.S.)
- Atara Biotherapeutics, Inc. (U.S.)
- Cellectis (France)
- Mustang Bio Inc. (U.S.)
- Immunocore (U.K.)
- TCR2 Therapeutics (U.S.)
- Celyad Oncology (Belgium)
- Bellicum Pharmaceuticals (U.S.)
- bluebird bio, Inc. (U.S.)
- Juno Therapeutics (U.S.)
- Poseida Therapeutics (U.S.)
- Cartherics (Australia)
- Eutilex (U.S.)
- Immunotech Biopharm (Australia)
- 3T Biosciences (U.S.)
- 4Cell Therapies S.A. (Poland)
- Aavocyte, Inc. (U.S.)
What are the Recent Developments in Global Cell-Based Immunotherapy Market?
- In May 2025, early clinical data for SENTI‑202 were confirmed as encouraging, with positive preliminary outcomes reported from a Phase 1 trial against relapsed/refractory hematologic cancers, reinforcing momentum for next‑generation cell‑based immunotherapies and demonstrating clinical feasibility beyond autologous CAR‑T approaches
- In April 2025, interim clinical trial results for SENTI‑202, a first‑in‑class off‑the‑shelf, logic‑gated CAR natural killer (CAR‑NK) cell therapy, showed complete remissions in relapsed or refractory acute myeloid leukemia (AML) patients, indicating strong potential for broader applicability of allogeneic cell therapies
- In August 2024, afamitresgene autoleucel (Tecelra) received FDA accelerated approval as the first engineered T‑cell receptor (TCR) therapy for unresectable or metastatic synovial sarcoma, representing a landmark for cell therapy in treating solid tumors beyond traditional CAR‑T hematologic applications
- In April 2024, the FDA expanded the indication for CAR‑T therapies such as liso‑cel (Breyanzi) and ide‑cel (Idecabtagene vicleucel), enabling broader treatment use in chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and earlier lines of multiple myeloma, significantly increasing patient access to these cell‑based treatments
- In February 2024, the U.S. Food and Drug Administration (FDA) granted accelerated approval to lifileucel (Amtagvi), the first tumor‑infiltrating lymphocyte (TIL) immunotherapy for adults with unresectable or metastatic melanoma previously treated with checkpoint inhibitors, marking the first FDA‑approved cellular therapy specifically for a solid tumor indication
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.