Global Cell Viability Assays Market
Market Size in USD Billion
CAGR :
% 
 USD
2.39 Billion
USD
4.21 Billion
2024
2032
USD
2.39 Billion
USD
4.21 Billion
2024
2032
| 2025 –2032 | |
| USD 2.39 Billion | |
| USD 4.21 Billion | |
|
|
|
|
Cell Viability Assays Market Size
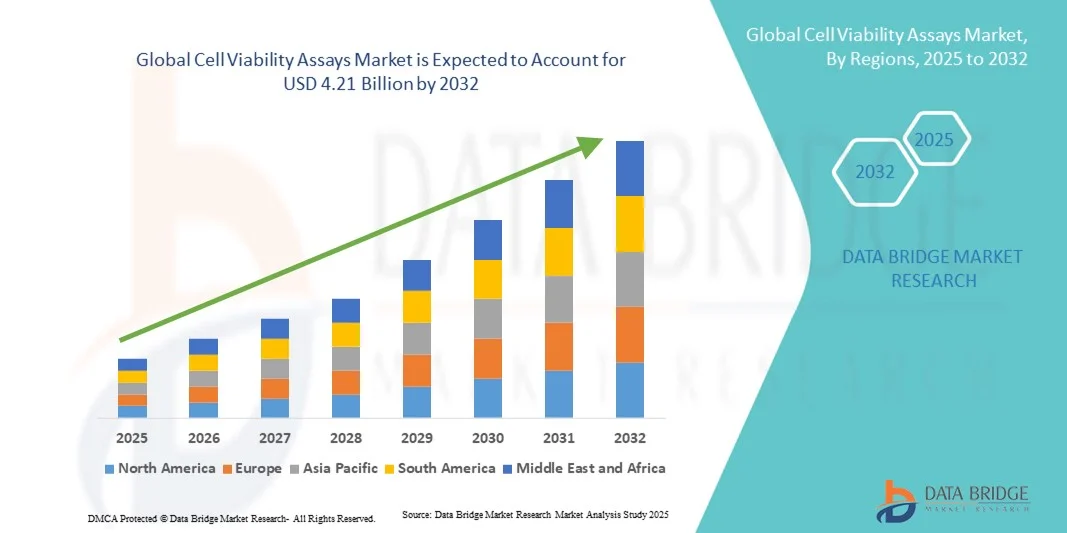
- The global cell viability assays market size was valued at USD 2.39 billion in 2024 and is expected to reach USD 4.21 billion by 2032, at a CAGR of 7.34% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced cell-based research techniques and technological progress in high-throughput screening, leading to enhanced precision and efficiency in biological and pharmaceutical studies
- Rising demand for reliable, sensitive, and reproducible assays in drug discovery, cancer research, stem cell studies, and toxicology testing is driving the adoption of Cell Viability Assays solutions, thereby significantly boosting the industry’s growth
Cell Viability Assays Market Analysis
- The Cell Viability Assays market encompasses solutions and technologies used to evaluate the health, proliferation, and survival of cells in various research and clinical applications, serving as a critical tool in drug discovery, cancer research, and toxicology studies
- The market growth is further driven by rising demand for sensitive, reproducible, and cost-effective assays in pharmaceutical, biotechnology, and academic research, accelerating the adoption of cell viability assays solutions and significantly boosting industry expansion
- North America dominated the cell viability assays market with the largest revenue share of 46.3% in 2024, driven by the presence of leading pharmaceutical and biotechnology companies, advanced research infrastructure, and strong R&D initiatives
- Asia-Pacific is expected to be the fastest-growing region in the cell viability assays market during the forecast period, projected to register a CAGR owing to rising biotechnology research, increasing adoption of automated assay platforms, and expanding clinical research activities
- The MTT Assay segment dominated the largest market revenue share of 41.5% in 2024, driven by its extensive use in cytotoxicity studies and drug screening applications
Report Scope and Cell Viability Assays Market Segmentation
|
Attributes |
Cell Viability Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cell Viability Assays Market Trends
Enhanced Focus on Automation and High-Throughput Screening
- A significant and accelerating trend in the global cell viability assays market is the increasing adoption of automation and high-throughput screening technologies, which is enhancing the efficiency, reproducibility, and scalability of cellular assays
- For instance, advanced robotic liquid handling systems and automated plate readers are being integrated with cell viability platforms, allowing laboratories to process large sample volumes with minimal human intervention. Similarly, imaging-based high-content screening systems enable simultaneous analysis of multiple cellular parameters, providing more comprehensive insights into cell health and behavior
- Integration of laboratory information management systems (LIMS) with cell viability platforms facilitates streamlined data acquisition, storage, and analysis, reducing the risk of manual errors and enabling faster decision-making in research and clinical environments
- The trend towards miniaturization of assays and multiplexing is also increasing, allowing multiple cell types or experimental conditions to be tested simultaneously, which optimizes reagent usage and reduces operational costs
- Companies such as Promega Corporation and Thermo Fisher Scientific are developing automated and high-throughput cell viability solutions with customizable assay protocols and advanced detection systems, supporting both academic research and industrial applications
- The demand for more efficient, reliable, and scalable cell viability assay platforms is growing rapidly across pharmaceutical, biotechnology, and clinical research sectors, as users increasingly prioritize time-saving and standardized workflows
Cell Viability Assays Market Dynamics
Driver
Rising Demand for High-Throughput and Reliable Assay Platforms
- The increasing demand for high-throughput and accurate cell viability measurements in drug discovery, toxicology, and disease modeling is a significant driver for the market
- For instance, in April 2024, Promega Corporation launched its next-generation CellTiter-Glo 3D Cell Viability Assay optimized for automated high-throughput screening of 3D cell models. Such product innovations are expected to accelerate the growth of the Cell Viability Assays market during the forecast period
- As pharmaceutical and biotechnology companies expand their research pipelines, there is a heightened need for robust, reproducible, and scalable assays that can handle increasing sample volumes efficiently
- In addition, the adoption of multi-parametric and multiplexed assay formats allows researchers to simultaneously evaluate cell viability, proliferation, and cytotoxicity, providing comprehensive insights in less time
- Growing emphasis on reducing experimental variability and improving assay sensitivity is further driving the adoption of advanced cell viability platforms in both research and clinical laboratories
Restraint/Challenge
High Costs, Technical Complexity, and Regulatory Constraints
- The relatively high cost of advanced cell viability assay kits, reagents, and automated instrumentation poses a challenge for smaller research labs and institutions, particularly in developing regions
- For instance, sophisticated imaging-based or luminescence-based platforms often require specialized equipment and training, which may limit adoption among resource-constrained laboratories
- Technical complexity and the need for skilled personnel to handle automated systems or high-content screening platforms can hinder broader deployment
- While miniaturized and multiplexed assays reduce reagent usage and operational time, the initial investment and calibration requirements remain significant
- Limited compatibility across assay platforms and varying sensitivity of reagents for different cell types can create challenges for researchers in standardizing protocols
- Stringent regulatory requirements for clinical and pharmaceutical research increase the validation and documentation burden, delaying assay adoption and implementation
- The need for continuous software updates, instrument maintenance, and calibration in high-throughput systems adds to operational costs and complexity
- Issues such as variability in assay performance across different laboratories, interference from test compounds, or sensitivity to environmental conditions can affect reproducibility and reliability of results
- Short shelf-life of some reagents and storage sensitivity further complicate logistics, especially for labs in regions with limited cold-chain infrastructure
- Overcoming these challenges through cost-effective assay kits, standardized protocols, technical training, and strong vendor support will be crucial for sustained growth in the global Cell Viability Assays market
Cell Viability Assays Market Scope
The market is segmented on the basis of assay type, application, end-user, and technology.
- By Assay Type
On the basis of assay type, the Cell Viability Assays market is segmented into MTT Assay, Trypan Blue Exclusion Assay, ATP Assay, and Others. The MTT Assay segment dominated the largest market revenue share of 41.5% in 2024, driven by its extensive use in cytotoxicity studies and drug screening applications. Its reliability, reproducibility, and cost-effectiveness make it a preferred choice among pharmaceutical companies and academic researchers. The segment also benefits from validation in diverse cell types and compatibility with both adherent and suspension cultures. Growing adoption in preclinical testing, compound screening, and cell proliferation studies continues to reinforce its dominance. Moreover, integration with high-throughput automated platforms enhances productivity and ensures consistent results. MTT Assays remain a staple in laboratories due to well-established protocols, robust data outputs, and broad applicability in both academic and industrial research settings.
The ATP Assay segment is expected to witness the fastest CAGR of 22.3% from 2025 to 2032, fueled by its high sensitivity, rapid detection capabilities, and suitability for diverse cell types, including primary and stem cells. The segment benefits from the increasing demand for real-time quantitative assessment of cell viability and metabolic activity. Advancements in luminescence-based ATP detection kits, automation integration, and high-throughput compatibility further accelerate adoption. ATP Assays are increasingly used in drug development, toxicity evaluation, and regenerative medicine research due to their precise measurement of cellular energy states. Rising investment in cell-based assays for novel therapeutics and the focus on scalability support this rapid market growth. Enhanced reproducibility, ease of multiplexing, and compatibility with modern detection instruments position ATP Assays as a key growth driver.
- By Application
On the basis of application, the Cell Viability Assays market is segmented into Drug Discovery & Development, Cancer Research, Stem Cell Research, and Toxicology Studies. Drug Discovery & Development dominated the largest revenue share of 38.7% in 2024, driven by the growing need for efficient screening of new chemical entities and biologics. This application is critical for preclinical evaluation, enabling the identification of cytotoxic effects, optimal dosing, and candidate selection. Pharmaceutical and biotechnology companies rely heavily on these assays to streamline pipelines, reduce late-stage failures, and ensure reproducible results. High-throughput screening, combinatorial chemistry, and translational research further enhance the segment’s market leadership. Regulatory compliance, standardization, and robust assay platforms reinforce its continued dominance. The rising focus on targeted therapies, monoclonal antibodies, and personalized medicine sustains strong demand.
Stem Cell Research is anticipated to witness the fastest CAGR of 21.8% from 2025 to 2032, driven by increasing emphasis on regenerative medicine, cell therapy, and personalized treatment strategies. Cell viability assessment is crucial for stem cell proliferation, differentiation, and transplantation studies, boosting assay adoption. Integration with automated platforms, high-content imaging, and advanced analytical tools enhances throughput and accuracy. Growing funding for stem cell research, innovative therapeutic applications, and collaborations with biotech firms accelerate market penetration. Assay sensitivity, reproducibility, and compatibility with complex stem cell models support fast growth. Increasing focus on preclinical validation and quality control of stem cell products also drives adoption.
- By End-User
On the basis of end-user, the Cell Viability Assays market is segmented into Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Contract Research Organizations (CROs), and Hospitals & Diagnostic Centers. Pharmaceutical & Biotechnology Companies accounted for the largest market revenue share of 42.6% in 2024, owing to extensive R&D activities and the demand for robust, scalable assays to accelerate drug development. High-throughput screening, translational research, and preclinical studies ensure continuous reliance on cell viability assays. Companies benefit from established workflows, standardized protocols, and reproducibility in various assay formats. Investments in automated systems, integration with bioinformatics, and focus on personalized medicine reinforce their market leadership. Rising demand for efficient compound screening, bioprocess optimization, and regulatory compliance further strengthen their dominance. Long-term collaborations with academic institutions and CROs continue to expand capabilities and applications.
CROs are expected to witness the fastest CAGR of 23.0% from 2025 to 2032, fueled by increasing outsourcing of preclinical and toxicology studies to specialized service providers. Rising demand for flexible, scalable assay solutions and high reproducibility drives growth. Collaborations with pharmaceutical and biotech firms for contract-based research enhance market penetration. CROs benefit from advanced automation, high-throughput screening compatibility, and regulatory compliance, ensuring reliable data outputs. Expansion of global clinical trial networks and the need for cost-efficient services accelerate adoption. Demand for high-quality assay services in emerging markets further supports rapid growth. CROs play a pivotal role in bridging research innovation and commercial product development.
- By Technology
On the basis of technology, the Cell Viability Assays market is segmented into Colorimetric, Fluorometric, Luminescent, and Others. Colorimetric assays dominated the largest market revenue share of 40.3% in 2024, owing to their simplicity, cost-effectiveness, and reproducibility for routine cytotoxicity and viability testing. They are widely used in both academic and industrial laboratories, supported by well-established protocols. Integration with automated platforms and multi-well plate readers enhances throughput and reliability. Colorimetric assays offer robust data for regulatory submissions and preclinical studies, sustaining their dominant position. Broad applicability across drug discovery, cancer research, and toxicology studies further strengthens market leadership.
Luminescent assays are expected to witness the fastest CAGR of 22.7% from 2025 to 2032, driven by high sensitivity, broad dynamic range, and the ability to detect low cell numbers. Advancements in luminescent substrates, integration with automated high-throughput screening platforms, and compatibility with advanced imaging systems fuel growth. Rising adoption in drug discovery, stem cell research, and personalized medicine applications accelerates expansion. The need for precise, reproducible, and rapid readouts in complex assay formats positions luminescent technology as the fastest-growing segment. Continuous improvements in assay robustness, multiplexing capabilities, and user-friendly formats further enhance adoption.
Cell Viability Assays Market Regional Analysis
- North America dominated the cell viability assays market with the largest revenue share of 46.3% in 2024, driven by the presence of leading pharmaceutical and biotechnology companies, advanced research infrastructure, and strong R&D initiatives
- The region benefits from well-established laboratories, high adoption of automated assay platforms, and a skilled workforce supporting both academic and industrial research
- Rapid advancements in assay technologies and the growing need for high-throughput screening in drug discovery and preclinical studies are key factors contributing to the market's growth
U.S. Cell Viability Assays Market Insight
The U.S. cell viability assays market captured the largest revenue share within North America in 2024, fueled by increasing investments in biopharmaceutical R&D, expansion of contract research organizations (CROs), and advancements in automated and high-yield assay platforms. The adoption of innovative assay technologies in pharmaceutical companies and research institutes, coupled with government support for life sciences research, continues to drive market expansion. The demand for precise and reliable cell viability data in drug discovery and translational research further reinforces the U.S.’s market dominance.
Europe Cell Viability Assays Market Insight
The Europe cell viability assays market is projected to expand at a substantial CAGR during the forecast period, driven by rising investment in biotechnology and life sciences research, stringent regulatory standards for drug development, and the increasing presence of CROs. Germany, France, and the U.K. are key contributors, leveraging their advanced research infrastructure and adoption of automated assay platforms across pharmaceutical and academic research sectors.
U.K. Cell Viability Assays Market Insight
The U.K. cell viability assays market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by government funding for biotechnology research, increasing clinical trial activities, and the integration of automated and high-throughput assay technologies in pharmaceutical and academic research. Life sciences companies and academic institutes are key drivers of this growth, focusing on improved drug discovery and translational research capabilities.
Germany Cell Viability Assays Market Insight
The Germany cell viability assays market is expected to expand at a considerable CAGR during the forecast period, fueled by rising investments in biotechnology research, advanced infrastructure for clinical studies, and the growing emphasis on personalized medicine. Pharmaceutical and biopharmaceutical companies are increasingly adopting high-throughput cell viability assays for efficient R&D operations.
Asia-Pacific Cell Viability Assays Market Insight
The Asia-Pacific cell viability assays market is poised to be the fastest-growing region during the forecast period, projected to register a high CAGR owing to rising biotechnology research, increasing adoption of automated assay platforms, and expanding clinical research activities. Countries such as China, Japan, and India are emerging as key pharmaceutical and biotechnology hubs, supported by government initiatives and growing presence of CROs.
Japan Cell Viability Assays Market Insight
The Japan cell viability assays market is gaining momentum due to advanced pharmaceutical research capabilities, technological adoption in laboratories, and rising clinical research activities. Government support for biotechnology innovation, coupled with high adoption of automated and high-throughput assay platforms, is contributing to sustained growth.
China Cell Viability Assays Market Insight
The China cell viability assays market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by the expansion of biopharmaceutical R&D infrastructure, government support for biotechnology initiatives, and increasing adoption of automated and CHO-based assay platforms in clinical and industrial research. Strong growth in academic research institutions and contract research organizations further reinforces market expansion.
Cell Viability Assays Market Share
The Cell Viability Assays industry is primarily led by well-established companies, including:
• Thermo Fisher Scientific Inc (U.S.)
• Merck KGaA (Germany)
• Lonza Group Ltd. (Switzerland)
• Bio-Rad Laboratories, Inc. (U.S.)
• Promega Corporation (U.S.)
• PerkinElmer, Inc. (U.S.)
• Sigma-Aldrich (U.S.)
• Agilent Technologies, Inc. (U.S.)
• Danaher Corporation (U.S.)
• GE Healthcare Life Sciences (U.K.)
Latest Developments in Global Cell Viability Assays Market
- In October 2021, Nanolive launched the LIVE Cell Death Assay (LCDA), a first-in-class cytotoxicity analysis tool providing dynamic, label-free live cell data. Developed using state-of-the-art machine learning, the LCDA can distinguish between living cells, apoptosis, and necrosis, delivering 13 viability and death-related metrics with a single click
- In September 2022, Nanolive introduced the LIVE Cell Death Assay V1.3, an updated version of their cytotoxicity analysis tool. This release continued to offer dynamic, label-free live cell data, enhancing the study of cell death dynamics
- In August 2023, Nanolive released the LIVE Cytotoxicity Assay V1.0, further expanding their suite of tools for label-free live cell imaging. This assay aimed to provide researchers with more options for studying cell health and death in real-time
- In July 2025, Promega's RealTime-Glo MT Cell Viability Assay was cited in a study on engineering novel CRISPRi repressors for highly efficient mammalian gene regulation. The assay was used to quantify relative cell numbers, highlighting its application in gene regulation research
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.