Global Centronuclear Myopathies Drug Market
Market Size in USD Million
CAGR :
% 
 USD
240.66 Million
USD
390.25 Million
2024
2032
USD
240.66 Million
USD
390.25 Million
2024
2032
| 2025 –2032 | |
| USD 240.66 Million | |
| USD 390.25 Million | |
|
|
|
|
Centronuclear Myopathies Drug Market Size
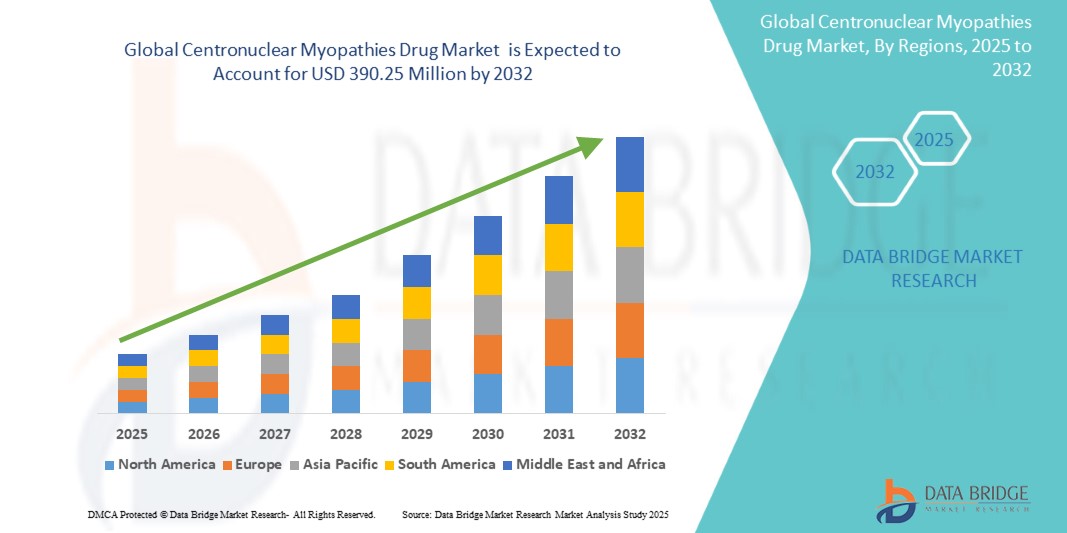
- The Global Centronuclear Myopathies Drug Market size was valued at USD 240.66 million in 2024 and is expected to reach USD 390.25 Million by 2032, at a CAGR of 6.4% during the forecast period
- This growth is driven by increasing R&D activity targeting rare neuromuscular disorders, rising global awareness, and advancements in gene-based and enzyme-targeted therapies.
Centronuclear Myopathies Drug Market Analysis
- Centronuclear myopathies (CNMs) are rare congenital muscle disorders that impair muscle strength and function due to abnormal positioning of cell nuclei. Increasing regulatory support for orphan drugs and early diagnosis through newborn screening are significantly enhancing treatment accessibility and awareness
- The demand for these microscopes is significantly driven by the increasing prevalence of age-related eye conditions and advancements in surgical techniques
- North America is expected to dominate the Centronuclear Myopathies Drugs market due to early access to clinical trials and regulatory approvals
- Asia-Pacific is expected to be the fastest growing region in the Centronuclear Myopathies Drug market during the forecast period due to growth through collaborative research initiatives
- Antisense oligonucleotides and enzyme replacement therapies are gaining traction as the standard of care for certain CNM subtypes, with several candidates in late-phase clinical development
Report Scope and Centronuclear Myopathies Drug Market Segmentation
|
Attributes |
Centronuclear Myopathies Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Centronuclear Myopathies Drug Market Trends
“Emerging Gene Therapies and Strategic Collaborations”
- One prominent trend in the Centronuclear Myopathies drug market is the rising focus on gene therapies and targeted molecular treatments to address the underlying genetic causes of the disease.
- These innovations, such as Dynacure’s DYN101 and ARMGO Pharma’s ARM210, are being developed through strategic partnerships and offer the potential to significantly improve clinical outcomes by modifying disease progression at the molecular level.
- For instance, in 2024, advancements in high-resolution imaging and next-generation sequencing technologies significantly improved the ability to diagnose Centronuclear Myopathies (CNM) at earlier stages, allowing researchers and clinicians to better understand genotype-phenotype correlations.
- These innovations are transforming the management of CNM by supporting earlier intervention and personalized treatment approaches, thereby driving demand for next-generation therapeutics and targeted drug development.
Centronuclear Myopathies Drug Market Dynamics
Driver
“Advancements in Genetic Therapies and Regulatory Incentives”
- The development of gene therapies and antisense platforms is accelerating treatment availability for CNMs. Regulatory bodies are actively fast-tracking these innovations under orphan drug and rare pediatric designations.
- As advancements in genetic therapies accelerate, particularly with the development of AAV-based gene therapies and exon-skipping techniques, there is growing optimism for disease-modifying treatments for Centronuclear Myopathies (CNM).
- In response, regulatory agencies such as the FDA and EMA have introduced incentives like Orphan Drug Designation and accelerated approval pathways, significantly boosting investment and R&D activity in the CNM drug market
For instance,
- For instance, in 2024, the FDA granted breakthrough therapy status to an antisense candidate developed for X-linked CNM, boosting investor interest and development speed.
- As a result of advancements in genetic therapies and growing regulatory support, there is a significant increase in the development and demand for Centronuclear Myopathies (CNM) drugs, driven by the rising identification of CNM cases through improved genetic screening and increased availability of targeted treatment options
Opportunity
“Increasing Global Patient Advocacy and Awareness Initiatives”
- Rare disease foundations and global neuromuscular alliances are helping spread awareness and influence research priorities. Patient registries and outreach programs have expanded diagnostic reach in underserved regions
- Growing global patient advocacy and awareness initiatives are playing a pivotal role in accelerating early diagnosis and access to treatment for Centronuclear Myopathies (CNM), as organizations work to educate both the public and healthcare professionals about the disease.
- • Additionally, these initiatives are fostering collaboration among researchers, pharmaceutical companies, and regulatory bodies, helping to drive funding, clinical trial participation, and the development of more effective CNM therapies.
For instance,
- For example, in 2025, the Myotubular Trust and European CNM Consortium launched an awareness campaign and biobank platform to centralize patient data and support new therapy trials
- Furthermore, advocacy groups are instrumental in connecting patients with emerging therapies and support networks, empowering families and influencing policy to prioritize rare neuromuscular disease research and treatment access.
Restraint/Challenge
“Limited Access and High Treatment Costs in Low-Income Regions”
- Despite global recognition, many patients in lower-income countries face delays in diagnosis and lack access to treatment due to cost and absence of local expertise.
- Advanced therapies for Centronuclear Myopathies (CNM), such as gene therapies and specialized biologics, often come with high development and distribution costs, making them financially inaccessible for many patients in low-income regions
- This substantial cost barrier limits access to cutting-edge treatments and clinical trials, leading to delayed diagnoses, suboptimal care, and continued reliance on supportive rather than curative therapies in underserved populations.
For instance,
- A 2023 study in The Lancet Neurology reported that 68% of CNM patients in Southeast Asia were undiagnosed or misdiagnosed due to absence of neuromuscular specialists and genetic testing access
- Consequently, such limitations contribute to significant disparities in the diagnosis and treatment of Centronuclear Myopathies (CNM), restricting patient access to emerging genetic therapies and specialized care, and ultimately hindering the global growth and equitable development of the CNM drug market
Centronuclear Myopathies Drug Market Scope
The market is segmented on the basis drug type, route of administration, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the Antisense Oligonucleotides is projected to dominate the market with a largest share in drug type segment
The Antisense Oligonucleotides segment is expected to dominate the Centronuclear Myopathies Drug market with the largest share of 56.22% in 2025 due to its targeted mechanism of action and growing demand for precision medicine in rare neuromuscular disorders. As advancements in genetic research continue, antisense therapies are showing promising clinical outcomes in modifying the expression of mutated genes associated with CNM. Increasing awareness, early diagnosis through genetic screening, and expanding clinical trials further contribute to the segment’s projected market leadership.
The Oral is expected to account for the largest share during the forecast period in technology market
In 2025, the Oral segment is expected to dominate the market with the largest market share of 51.31% due to the convenience of administration, improved patient compliance, and ongoing development of orally delivered small molecules targeting neuromuscular pathways. As research progresses, oral formulations are becoming increasingly viable for managing symptoms and modifying disease progression, particularly in pediatric and adult CNM populations. Additionally, growing demand for non-invasive treatment options supports the segment's projected dominance..
Centronuclear Myopathies Drug Market Regional Analysis
“North America Holds the Largest Share in the Centronuclear Myopathies Drug Market”
- North America is expected to dominate the Centronuclear Myopathies (CNM) drug market, driven by its advanced healthcare infrastructure, high adoption of innovative genetic therapies, and the strong presence of key pharmaceutical and biotechnology companies focused on rare neuromuscular diseases.
- The U.S. holds a significant share due to increasing demand for precision medicine, heightened awareness and diagnosis of CNM through genetic screening, and continuous advancements in gene-based treatments.
- The availability of well-established reimbursement policies for rare disease treatments and significant investments in research & development by leading pharmaceutical companies further strengthen the market.
- Additionally, the growing emphasis on rare disease therapies and the rising number of clinical trials dedicated to CNM is fueling market growth across the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Centronuclear Myopathies Drug Market”
- The Asia-Pacific region is expected to witness the highest growth rate in the Centronuclear Myopathies (CNM) drug market, driven by rapid advancements in healthcare infrastructure, increasing awareness about rare neuromuscular diseases, and rising participation in clinical trials.
- Countries such as China, India, and Japan are emerging as key markets due to the growing recognition of CNM and other rare genetic disorders, alongside the expanding focus on genetic screening and personalized treatments.
- Japan, with its advanced medical technology and strong healthcare system, remains a crucial market for CNM drug development and research. The country continues to lead in the adoption of cutting-edge therapies and innovative treatment approaches for rare diseases like CNM.
- China and India, with their large populations and increasing incidence of genetic disorders, are witnessing increased government and private sector investments in rare disease drug development. The growing presence of global pharmaceutical companies and improvements in healthcare access are further contributing to market growth.
Centronuclear Myopathies Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Audentes Therapeutics (United States)
- Dynacure (France)
- Astellas Pharma Inc. (Japan)
- Valerion Therapeutics (United States)
- Biogen Inc. (HQ: United States)
- Ionis Pharmaceuticals (United States)
- Sarepta Therapeutics (United States)
- Genethon (France)
Latest Developments in Global Centronuclear Myopathies Drug Market
- In January 2025, Dynacure presented Phase I/II study results for DYN101, its antisense drug candidate for CNM, at the World Muscle Society conference.
- In February 2025, Astellas launched a CNM clinical trial registry platform to consolidate global patient participation across centers.
- In March 2025, Biogen signed a strategic agreement with Genethon for preclinical CNM gene therapy development.
- In April 2025, Ionis Pharmaceuticals initiated a global trial evaluating systemic administration of a next-gen antisense platform for rare myopathies.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.