Global Cerebral Adrenoleukodystrophy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
6.48 Billion
USD
12.92 Billion
2025
2033
USD
6.48 Billion
USD
12.92 Billion
2025
2033
| 2026 –2033 | |
| USD 6.48 Billion | |
| USD 12.92 Billion | |
|
|
|
|
Cerebral Adrenoleukodystrophy Treatment Market Size
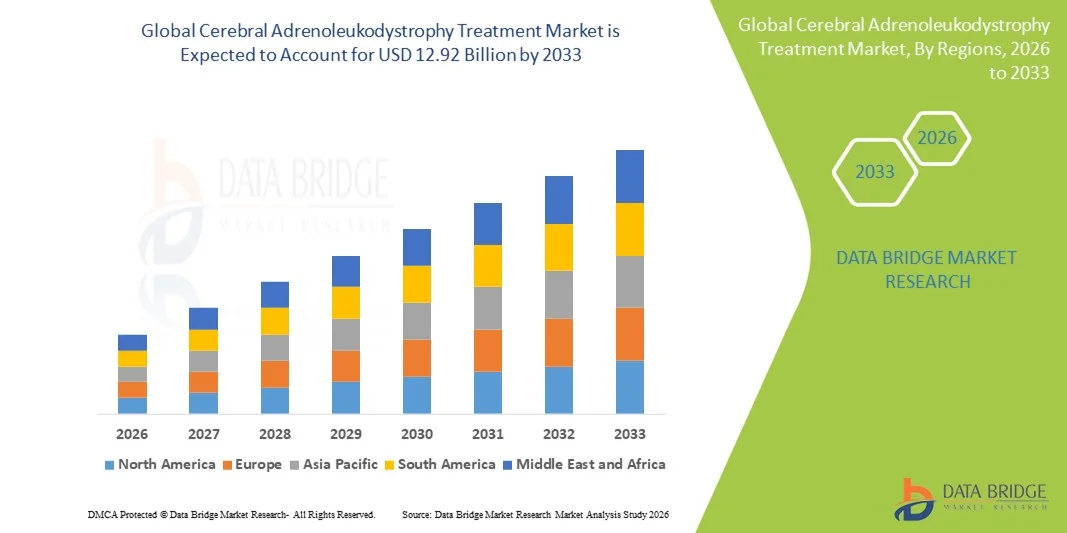
- The global cerebral adrenoleukodystrophy treatment market size was valued at USD 6.48 billion in 2025 and is expected to reach USD 12.92 billion by 2033, at a CAGR of 9.00% during the forecast period
- The market growth is largely fueled by advancements in gene therapy and stem cell transplantation, targeting the underlying ABCD1 gene defect, along with increasing R&D investments in rare disease treatments
- Furthermore, expanded newborn screening programs, rising awareness among healthcare providers, and supportive regulatory policies for orphan drugs are driving earlier diagnosis and treatment adoption. These converging factors are accelerating the uptake of disease-modifying therapies, thereby significantly boosting the industry's growth
Cerebral Adrenoleukodystrophy Treatment Market Analysis
- Cerebral adrenoleukodystrophy (CALD) treatments, including stem cell transplant, gene therapy, corticosteroids, Lorenzo’s oil, and other supportive therapies, are increasingly vital components of rare disease management due to their potential to halt or slow neurological decline and improve patient outcomes
- The escalating demand for cerebral adrenoleukodystrophy treatments is primarily fueled by advancements in gene therapy, wider adoption of newborn screening programs, and growing awareness of the disorder among healthcare providers and caregivers
- North America dominated the cerebral adrenoleukodystrophy treatment market with the largest revenue share of 45.2% in 2025, characterized by advanced healthcare infrastructure, strong rare-disease R&D investments, and supportive orphan-drug regulatory frameworks, with the U.S. leading in adoption of stem cell transplant and emerging gene therapies
- Asia-Pacific is expected to be the fastest-growing region in the cerebral adrenoleukodystrophy treatment market during the forecast period due to increasing healthcare access, rising genetic testing capabilities, and improved availability of advanced therapies in countries such as China, Japan, and India
- Stem cell transplant segment dominated the cerebral adrenoleukodystrophy treatment market in 2025 with a market share of 48.5%, driven by its established efficacy in early-stage cerebral ALD
Report Scope and Cerebral Adrenoleukodystrophy Treatment Market Segmentation
|
Attributes |
Cerebral Adrenoleukodystrophy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cerebral Adrenoleukodystrophy Treatment Market Trends
Advancements in Gene Therapy and Disease-Modifying Treatments
- A significant and accelerating trend in the global cerebral adrenoleukodystrophy treatment market is the rapid development and clinical adoption of gene therapy approaches targeting the ABCD1 gene, offering potential long-term correction of the underlying metabolic defect
- For instance, lentiviral-based ex vivo gene therapy is being increasingly studied and applied in early-stage cerebral ALD patients, aiming to halt disease progression before severe neurological damage occurs
- Gene therapy and novel disease-modifying treatments enable personalized care plans by potentially reducing the need for repeated interventions and improving neurological outcomes, allowing patients to maintain higher quality of life
- The integration of gene therapy with early diagnosis through expanded newborn screening programs facilitates timely intervention, improving long-term prognosis and reducing disease burden on families and healthcare systems
- This trend towards innovative, targeted, and potentially curative therapies is fundamentally reshaping patient expectations and standard-of-care practices, encouraging pharmaceutical companies to invest heavily in advanced treatment development
- The demand for gene therapy and other disease-modifying treatments is growing rapidly across both developed and emerging markets, as stakeholders increasingly prioritize effective, long-term solutions over purely symptomatic management
Cerebral Adrenoleukodystrophy Treatment Market Dynamics
Driver
Rising Awareness and Early Diagnosis through Newborn Screening
- The increasing awareness of cerebral adrenoleukodystrophy among healthcare providers, caregivers, and patient communities, coupled with the expansion of newborn screening programs, is a significant driver for the heightened demand for early intervention therapies
- For instance, in 2024, several U.S. states expanded newborn screening to include ALD, enabling identification of affected infants before clinical symptoms appear, thereby increasing the uptake of curative and disease-modifying treatments
- As early detection improves, treatment outcomes, especially from stem cell transplant or gene therapy, are substantially enhanced, driving physician recommendation and caregiver adoption
- Furthermore, global advocacy and educational initiatives are raising awareness in both developed and emerging countries, contributing to higher diagnosis rates and market growt
- The availability of therapies that can slow or halt disease progression, combined with the ability to intervene earlier, is encouraging healthcare systems to integrate CALD treatment into standard care pathways
- For instance, partnerships between patient advocacy groups and pharma companies are promoting early screening campaigns, further expanding treatment uptake
- Increasing government incentives for rare disease research and orphan drug development are providing financial and regulatory support, driving accelerated therapy approval and adoption
Restraint/Challenge
High Treatment Costs and Limited Access in Emerging Regions
- The substantial cost of advanced cerebral adrenoleukodystrophy therapies, including stem cell transplant and gene therapy, poses a significant barrier to broader market penetration, particularly in emerging economies
- For instance, the multi-step process of autologous stem cell transplant with gene modification can cost several hundred thousand dollars, limiting accessibility to only high-income patients or countries with reimbursement support
- Addressing affordability challenges through insurance coverage, government funding, and patient assistance programs is critical to expanding access and adoption of CALD treatments
- Furthermore, the need for specialized treatment centers with skilled clinicians and advanced infrastructure restricts availability, especially in regions lacking advanced healthcare facilities
- Overcoming these challenges through expanded infrastructure, policy support, and cost-reduction strategies will be vital for ensuring equitable access and sustained market growth
- For instance, limited awareness and training of healthcare providers in emerging countries further hinder early diagnosis and treatment initiation, reducing overall market penetration
- Supply chain constraints for specialized therapies, including gene-modified stem cells, can delay treatment delivery and limit adoption in regions without advanced logistics support
Cerebral Adrenoleukodystrophy Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, symptoms, end-users, and distribution channels.
- By Treatment
On the basis of treatment, the cerebral adrenoleukodystrophy treatment market is segmented into stem cell transplant, gene therapy, corticosteroids, Lorenzo’s oil, and others. The stem cell transplant segment dominated the market with the largest revenue share of 48.5% in 2025, driven by its proven efficacy in halting the progression of early-stage cerebral ALD. Stem cell transplant remains the standard of care for patients diagnosed before severe neurological damage occurs. Its adoption is supported by long-term clinical evidence, widespread physician familiarity, and inclusion in treatment guidelines. The segment’s dominance is further strengthened by increasing investments in stem cell banking and transplantation infrastructure across developed regions. Patients and caregivers often prefer stem cell transplant due to its potential to significantly improve survival and neurological outcomes. Additionally, the growing awareness of early diagnosis through newborn screening programs is increasing the demand for timely transplantation procedures.
The gene therapy segment is anticipated to witness the fastest growth from 2026 to 2033, driven by its potential as a one-time, disease-modifying therapy targeting the underlying ABCD1 gene defect. Gene therapy offers the promise of long-term correction of the metabolic disorder, reducing reliance on repeated interventions. Clinical successes in lentiviral-based gene therapy have raised adoption interest among healthcare providers and investors. The rising number of clinical trials, regulatory support for orphan drugs, and collaborations between biotech companies and academic institutions are accelerating the segment’s growth. Additionally, patient preference is shifting toward gene therapy due to the potential for improved quality of life and reduced long-term treatment burden. The increasing availability of specialized treatment centers capable of administering gene therapy further supports market expansion.
- By Diagnosis
On the basis of diagnosis, the cerebral adrenoleukodystrophy treatment market is segmented into blood tests, magnetic resonance imaging (MRI), eye exams, and skin biopsy. The blood tests segment dominated the market with the largest revenue share in 2025, due to its role in detecting elevated very long-chain fatty acids (VLCFA) and confirming ALD diagnosis quickly. Blood tests are minimally invasive, relatively cost-effective, and form the backbone of newborn screening programs worldwide. Early detection through blood tests facilitates timely treatment interventions, improving prognosis and long-term outcomes. The segment benefits from increasing awareness among pediatricians and rare disease specialists about the importance of biochemical screening. It is also supported by technological advances enabling faster and more accurate results. Blood testing adoption is higher in regions with robust public health programs and mandatory newborn screening policies.
The MRI segment is expected to witness the fastest growth from 2026 to 2033, as it enables precise monitoring of cerebral demyelination and disease progression in ALD patients. MRI provides detailed imaging of brain lesions, which is critical for determining treatment eligibility, particularly for stem cell transplant and gene therapy. The rising number of patients diagnosed early through blood tests drives MRI demand for follow-up evaluations. Advanced imaging protocols and AI-assisted MRI analysis are further boosting adoption. The increasing availability of MRI facilities in emerging regions is also supporting the rapid growth of this segment. Furthermore, healthcare providers increasingly rely on MRI to personalize treatment plans and monitor therapeutic efficacy, enhancing clinical outcomes.
- By Symptoms
On the basis of symptoms, the cerebral adrenoleukodystrophy treatment market is segmented into attention deficit disorder, behavior problems, hyperactivity, clumsiness, low blood sugar, eye pain, migraine, viral infections, and others. The behavior problems segment dominated in 2025 due to its prevalence in children with cerebral ALD and the need for early therapeutic intervention. Behavioral changes are often the first clinical indicators prompting diagnosis and treatment, driving demand for specialized therapies. Monitoring and managing these symptoms are critical for treatment planning and patient quality of life. Behavioral symptom recognition also influences early screening and physician referrals. Interventions targeting these symptoms are increasingly integrated with overall treatment strategies such as stem cell transplant or gene therapy. Caregivers play a key role in recognizing and reporting behavioral symptoms, further supporting the segment’s dominance.
The hyperactivity segment is expected to witness the fastest growth during the forecast period, as it often accompanies early-stage cerebral ALD and prompts early clinical attention. The rise in awareness among parents, teachers, and pediatricians regarding neurobehavioral signs is driving early diagnosis. Pharmaceutical companies are also developing therapies targeting symptomatic management alongside disease-modifying treatments. Hyperactivity monitoring in combination with biochemical and imaging diagnostics supports treatment customization. Digital health platforms and telemedicine are increasingly used for continuous monitoring, contributing to segment growth. The need for integrated care addressing both neurological and behavioral symptoms further fuels adoption.
- By End-Users
On the basis of end-users, the market is segmented into clinic, hospital, and others. The hospital segment dominated the market in 2025, due to the requirement for specialized infrastructure, skilled personnel, and comprehensive care for administering therapies such as stem cell transplant and gene therapy. Hospitals provide access to multidisciplinary teams including neurologists, hematologists, and genetic counselors, which is critical for effective treatment. They also offer post-treatment monitoring and management of complications. The dominance of hospitals is supported by insurance coverage and government reimbursement policies in developed regions. High patient trust and availability of advanced diagnostic and therapeutic facilities further reinforce hospital preference.
The clinic segment is expected to witness the fastest growth from 2026 to 2033, driven by increasing access to outpatient follow-ups, gene therapy counseling, and monitoring of mild to moderate symptom patients. Clinics are expanding services for genetic counseling and patient education, making them more attractive for routine management. The growth is also aided by telemedicine adoption, enabling remote consultations and follow-ups. Emerging regions with limited hospital access are increasingly relying on specialized clinics for early diagnosis and ongoing care. Clinics provide convenience, reduced costs, and personalized patient attention, supporting faster growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated in 2025 due to the controlled distribution of high-cost therapies such as stem cell transplant kits, gene therapy materials, and other specialized medicines. Hospital pharmacies ensure safe storage, proper handling, and compliance with regulatory requirements, which is critical for rare disease treatments. Integration with hospital treatment programs further strengthens this channel. Dominance is also reinforced by reimbursement systems that are tied to hospital-administered therapies.
The online pharmacy segment is expected to witness the fastest growth from 2026 to 2033, fueled by the increasing adoption of telemedicine, digital prescriptions, and direct-to-patient delivery of supportive therapies such as corticosteroids and Lorenzo’s oil. Online platforms enhance patient convenience, particularly in remote or underserved regions. The growth is supported by digital health integration and improved logistics networks. Patients and caregivers increasingly prefer online access for non-urgent, routine medications, enabling adherence to long-term therapy plans. Expansion of online pharmacies in emerging markets also contributes to segment growth.
Cerebral Adrenoleukodystrophy Treatment Market Regional Analysis
- North America dominated the cerebral adrenoleukodystrophy treatment market with the largest revenue share of 45.2% in 2025, characterized by advanced healthcare infrastructure, strong rare-disease R&D investments, and supportive orphan-drug regulatory frameworks, with the U.S. leading in adoption of stem cell transplant and emerging gene therapies
- Patients and healthcare providers in the region highly value early diagnosis through newborn screening, access to specialized treatments such as stem cell transplant and gene therapy, and comprehensive care from multidisciplinary hospital teams
- This widespread adoption is further supported by favorable orphan drug regulations, reimbursement support, and high awareness among physicians and caregivers, establishing North America as the leading region for CALD treatment adoption
U.S. Cerebral Adrenoleukodystrophy Treatment Market Insight
The U.S. cerebral adrenoleukodystrophy treatment market captured the largest revenue share of 50% in 2025 within North America, fueled by widespread adoption of newborn screening programs and advanced therapeutic options such as stem cell transplant and gene therapy. Patients and healthcare providers increasingly prioritize early intervention to halt neurological progression and improve long-term outcomes. The growing availability of specialized treatment centers, combined with strong reimbursement support and government incentives for rare diseases, further propels market growth. Moreover, increasing awareness among caregivers and pediatricians regarding early diagnosis is significantly contributing to the expansion of CALD treatments.
Europe Cerebral Adrenoleukodystrophy Treatment Market Insight
The Europe cerebral adrenoleukodystrophy treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising awareness of rare diseases and improved access to advanced therapies. Increasing healthcare expenditure and established rare disease frameworks in countries such as Germany, France, and Italy are fostering adoption of stem cell transplant and gene therapy. European patients and caregivers are also drawn to timely diagnosis, early intervention, and the availability of comprehensive care programs. The region is experiencing significant growth across hospitals and specialized clinics, with CALD treatments increasingly incorporated into national newborn screening and rare disease initiatives.
U.K. Cerebral Adrenoleukodystrophy Treatment Market Insight
The U.K. cerebral adrenoleukodystrophy treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by expanding newborn screening programs and a growing emphasis on rare disease management. Concerns regarding disease progression and neurological impairment are encouraging caregivers and healthcare providers to adopt early therapeutic interventions. The U.K.’s robust healthcare infrastructure, alongside increasing access to gene therapy and stem cell transplantation, is expected to continue stimulating market growth. Public awareness campaigns and patient advocacy programs are further promoting early diagnosis and treatment adoption.
Germany Cerebral Adrenoleukodystrophy Treatment Market Insight
The Germany cerebral adrenoleukodystrophy treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced diagnostic infrastructure and the availability of specialized treatment centers. Germany’s focus on rare disease research, coupled with strong healthcare reimbursement policies, promotes the adoption of stem cell transplant and gene therapy. The integration of multidisciplinary care teams ensures effective treatment planning and follow-up, supporting better patient outcomes. Additionally, growing awareness among healthcare providers and caregivers regarding early intervention drives increased uptake of CALD therapies.
Asia-Pacific Cerebral Adrenoleukodystrophy Treatment Market Insight
The Asia-Pacific cerebral adrenoleukodystrophy treatment market is poised to grow at the fastest CAGR during the forecast period, driven by rising healthcare access, expanding newborn screening initiatives, and increasing awareness of rare diseases in countries such as China, Japan, and India. Technological advancements and government initiatives promoting early diagnosis and rare disease treatment are boosting adoption. Moreover, the growing number of specialized treatment centers and increasing availability of gene therapy and stem cell transplant in select APAC countries are expanding access to CALD treatments.
Japan Cerebral Adrenoleukodystrophy Treatment Market Insight
The Japan cerebral adrenoleukodystrophy treatment market is gaining momentum due to the country’s advanced healthcare system, rising awareness of rare genetic disorders, and demand for early intervention therapies. The growing number of newborn screening programs and specialized treatment centers is driving adoption of stem cell transplant and emerging gene therapies. Integration of CALD treatment programs with advanced diagnostic facilities ensures timely intervention and improved patient outcomes. Additionally, Japan’s focus on patient education and caregiver support further contributes to market expansion.
India Cerebral Adrenoleukodystrophy Treatment Market Insight
The India cerebral adrenoleukodystrophy treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing awareness of rare diseases, expanding healthcare infrastructure, and government-supported newborn screening programs. India is witnessing growing adoption of advanced therapies such as stem cell transplant and gene therapy in specialized centers. The push towards early diagnosis, along with improved affordability and accessibility of treatments, is driving market growth. Moreover, patient advocacy and rare disease initiatives are key factors propelling the uptake of CALD therapies in both residential and hospital settings.
Cerebral Adrenoleukodystrophy Treatment Market Share
The Cerebral Adrenoleukodystrophy Treatment industry is primarily led by well-established companies, including:
- bluebird bio, Inc. (U.S.)
- Minoryx Therapeutics SL (Spain)
- Poxel SA (France)
- Viking Therapeutics, Inc. (U.S.)
- MedDay Pharmaceuticals (France)
- Orpheris, Inc. (U.S.)
- Magenta Therapeutics, Inc. (U.S.)
- NeuroVia Biotech, Inc. (U.S.)
- Autobahn Therapeutics (U.S.)
- Taysha GTx (U.S.)
- Neurogene, Inc. (U.S.)
- SwanBio Therapeutics, Inc. (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Regenxbio Inc. (U.S.)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Neurogene Inc. (U.S.)
- Spur Therapeutics (U.S.)
- Neuraxpharm Group GmbH (Germany)
- Genetix Biotherapeutics, Inc. (U.S.)
- Alcresta Therapeutics, Inc. (U.S.)
What are the Recent Developments in Global Cerebral Adrenoleukodystrophy Treatment Market?
- In August 2025, the FDA mandated updated labeling for Skysona to reflect a significantly higher incidence of hematologic malignancies about 15% more than triple the earlier reported rate. The boxed warning, usage indications, and safety sections of the prescribing information have been revised accordingly
- In July 2025, Minoryx Therapeutics and Neuraxpharm announced that the EMA validated their Marketing Authorization Application (MAA) for leriglitazone (Nezglyal®), a once-daily oral therapy for pediatric and adult male patients with cerebral ALD
- In November 2024, the U.S. FDA announced an investigation into reports of serious blood cancers in patients treated with Skysona, a gene therapy for early, active CALD, following trial-era cases being diagnosed 14 to 92 months after administration
- In November 2023, Minoryx Therapeutics announced the first patient enrollments in its U.S. Phase 3 CALYX trial for leriglitazone, targeting adult males with cerebral ALD (cALD) who are ineligible or decline stem‑cell transplant, advancing toward regulatory approval
- In November 2023, NHS England published a policy to routinely commission allogeneic hematopoietic stem-cell transplant (allo-HSCT) for adult male cALD patients, formalizing wider access to this treatment in the U.K.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.