Global Cervical Cancer Drug Market
Market Size in USD Billion
CAGR :
% 
 USD
8.25 Billion
USD
11.96 Billion
2024
2032
USD
8.25 Billion
USD
11.96 Billion
2024
2032
| 2025 –2032 | |
| USD 8.25 Billion | |
| USD 11.96 Billion | |
|
|
|
|
Cervical Cancer Drug Market Size
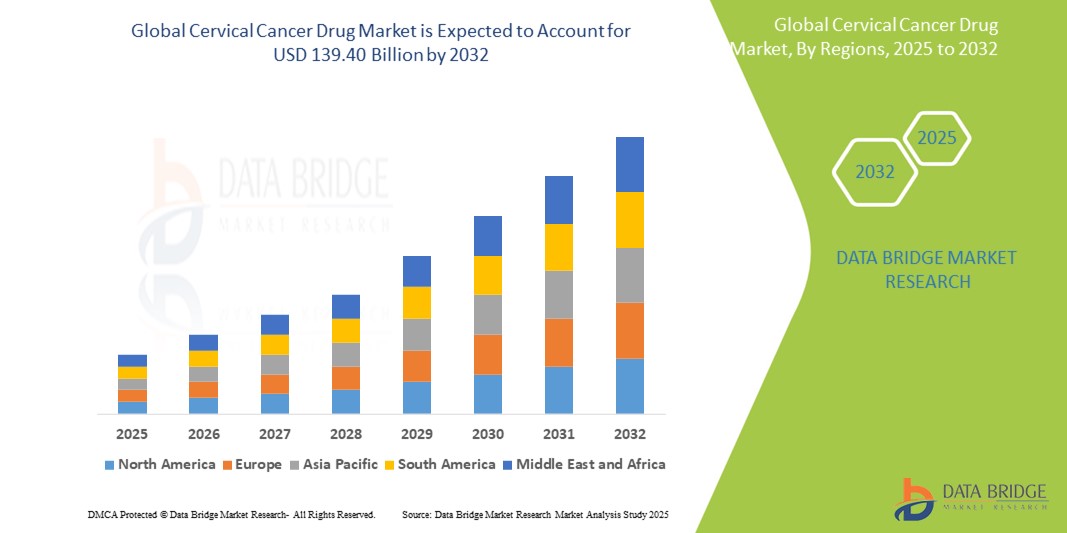
- The global cervical cancer drug market size was valued at USD 8.25 billion in 2024 and is expected to reach USD 11.96 billion by 2032, at a CAGR of 4.75% during the forecast period
- This growth is driven by increasing HPV-related cancer prevalence, rising demand for advanced immunotherapies, and growing awareness of cancer screening programs
Cervical Cancer Drug Market Analysis
- Cervical cancer is primarily caused by persistent infection with high-risk human papillomavirus (HPV) types. Drug treatment options include chemotherapy, immunotherapy, and targeted therapy, especially for late-stage or recurrent cervical cancer
- The market is being propelled by expanding access to cancer care, government vaccination initiatives, and rising investment in oncology R&D
- North America dominates the cervical cancer drug market with a market share of approximately 38.7%, supported by high awareness, robust screening programs, and access to advanced treatments.
- Asia-Pacific is projected to grow at the fastest pace and currently holds an estimated market share of 25.3%, driven by large patient pools and growing healthcare infrastructure.
- The immunotherapy segment is projected to grow at the highest CAGR of 13.7% during the forecast period due to increasing adoption of immune checkpoint inhibitors and novel monoclonal antibodies
Report Scope and Cervical Cancer Drug Market Segmentation
|
Attributes |
Cervical Cancer Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cervical Cancer Drug Market Trends
"Rise of Immune Checkpoint Inhibitors in Cervical Cancer Treatment"
- Immune checkpoint inhibitors, particularly PD-1/PD-L1 antibodies like pembrolizumab, have demonstrated superior clinical outcomes in patients with recurrent or metastatic cervical cancer compared to traditional chemotherapy
- For instance, in 2024, the U.S. FDA expanded the approval of pembrolizumab (Keytruda) for use in PD-L1-positive recurrent cervical cancer, based on the favorable results from the KEYNOTE-826 trial, which showed improved progression-free and overall survival
- There is an increasing number of clinical trials assessing the efficacy of immune checkpoint inhibitors in earlier stages of cervical cancer and in combination with other therapies like chemotherapy and radiation
- While initially available in high-income countries, these therapies are gradually being introduced into treatment guidelines and healthcare systems in middle-income markets due to their promising outcomes
- Immune checkpoint inhibitors are reshaping the treatment landscape of cervical cancer by offering targeted, effective options for advanced cases, with expanding regulatory approvals and increasing global uptake
Cervical Cancer Drug Market Dynamics
Driver
"Growing Incidence of HPV-Driven Cervical Cancer"
- According to WHO, cervical cancer remains a major global health burden, especially in low- and middle-income countries (LMICs), where healthcare infrastructure is limited and HPV vaccination rates are low
- For instance, Human papillomavirus (HPV) is responsible for over 95% of cervical cancer cases, making it a key focus of preventive and diagnostic strategies
- The rise in awareness and rollout of early screening programs such as Pap smears and HPV DNA testing is aiding in early-stage diagnosis, which improves survival rates
- International health bodies and governments are increasing investment in HPV vaccination programs, targeting young girls before the onset of sexual activity to reduce future disease burden
- The growing incidence of HPV-related cervical cancer continues to be a major driver for the cervical cancer drug market, propelling demand for both preventive and therapeutic interventions
Opportunity
"Expansion of Access Programs and Inclusion in Universal Health Coverage"
- Many countries are integrating cervical cancer care—including vaccination, screening, and treatment—into their universal health coverage (UHC) schemes, thereby improving access and affordability
- Partnerships with organizations such as Gavi, the Vaccine Alliance, have enabled resource-limited nations to procure HPV vaccines and essential cancer drugs at subsidized costs
- Collaborative funding models, particularly in sub-Saharan Africa, have allowed for better distribution networks and sustainable supply chains for cancer therapeutics
- As more patients are able to afford treatment through access programs, pharmaceutical companies see improved market penetration and brand loyalty in previously untapped regions.
- For instance, In 2023, Gavi partnered with several African governments to co-finance HPV vaccine procurement and cancer drug access
- The expansion of access programs and universal health coverage is creating significant opportunities for broader cervical cancer treatment dissemination, particularly in underserved populations
Restraint/Challenge
"Limited Drug Penetration in Rural and Low-Income Regions"
- Many rural areas lack oncology centers, diagnostic labs, and trained healthcare professionals, making it difficult to administer advanced therapies effectively
- Transport and storage challenges, especially for temperature-sensitive drugs like biologics, limit consistent drug availability in remote locations
- Even with subsidies, the high cost of immunotherapies and cancer diagnostics may remain out of reach for many individuals in low-income brackets
- Lack of awareness, stigma associated with cancer, and low health literacy further inhibit early diagnosis and treatment-seeking behavior in rural populations
- For instance, in 2023, a study published in Lancet Global Health cited lack of oncology centers and supply chain gaps as major hurdles in sub-Saharan Africa
- Limited access to healthcare infrastructure and affordability issues in rural and low-income regions continue to hinder the full market potential of cervical cancer treatments, requiring targeted policy and logistical interventions
The market is segmented on the basis of therapy type, route of administration, distribution channel, and end user
|
Segmentation |
Sub-Segmentation |
|
By Therapy Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the Immunotherapy is projected to dominate the market with a largest share in therapy type segment
In 2025, the immunotherapy segment is projected to dominate the market with the highest growth rate, holding a projected share of 38.6%, driven by better outcomes in advanced-stage patients and growing physician acceptance of biologics.
The Hospital segment expected to account for the largest share during the forecast period in end user market
In 2025, the hospitals are expected to account for the largest market share of 54.2% during the forecast period due to higher patient intake, availability of infusion centers, and access to multidisciplinary cancer care.
Cervical Cancer Drug Market Regional Analysis
“North America Holds the Largest Share in the Cervical Cancer Drug Market”
- North America dominates the cervical cancer drug market with a market share of approximately 38.7%, supported by high awareness, robust screening programs, and access to advanced treatments.
- The U.S. leads the region with an estimated 30.7% share, driven by strong regulatory support, early diagnosis rates, and the presence of major pharmaceutical players.
- North America, particularly the U.S. and Canada, has well-established public health campaigns and regular cervical cancer screening programs (Pap tests, HPV testing), leading to early detection and timely treatment
- The region benefits from a robust healthcare system with widespread access to cutting-edge treatments, including immunotherapies, precision medicine, and advanced diagnostic tools
- The U.S. leads the market due to the presence of the FDA, which fast-tracks approvals for innovative cancer therapies like pembrolizumab, allowing quicker clinical adoption
- Major global players such as Merck, Roche, and Bristol Myers Squibb are headquartered or heavily invested in North America, facilitating research, clinical trials, and faster commercial rollout of new drugs
“Asia-Pacific is Projected to Register the Highest CAGR in the Cervical Cancer Drug Market”
- Asia-Pacific is projected to grow at the fastest pace and currently holds an estimated market share of 25.3%, driven by large patient pools and growing healthcare infrastructure.
- China leads in the region with an estimated 8.7% share, supported by aggressive national vaccination programs and growing investments in oncology
- Countries across the region are prioritizing cervical cancer prevention and treatment through national programs. Several governments have introduced or expanded HPV vaccination initiatives and cancer registries, laying the groundwork for early diagnosis and improved treatment tracking
- India and China are experiencing a surge in collaborations between governments, NGOs, and pharmaceutical companies to improve women’s health. These partnerships are accelerating vaccine access, cancer screening, and the availability of affordable treatment options
- Many Asia-Pacific countries are increasing their oncology capacity through new cancer centers, enhanced medical training, and technology upgrades. This has led to greater adoption of targeted therapies and immunotherapies, particularly in urban centers
- Japan and South Korea are at the forefront of adopting cutting-edge treatments like immune checkpoint inhibitors. With advanced healthcare systems and strong R&D ecosystems, these countries are setting regional benchmarks for cervical cancer care
Cervical Cancer Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Merck & Co., Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- AstraZeneca (U.K.)
- Eli Lilly and Company (U.S.)
- Amgen Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Sanofi S.A. (France)
- Takeda Pharmaceutical Co., Ltd. (Japan)
- Biocon Limited (India)
- Seagen Inc. (U.S.)
Latest Developments in Global Cervical Cancer Drug Market
- In January 2025, Roche announced positive Phase III trial results for a new bispecific antibody targeting cervical tumor microenvironment, showing a 27% improvement in progression-free survival over standard care
- In October 2024, GSK received EMA approval for its novel HPV-targeted immunotherapy for second-line cervical cancer treatment, expanding its oncology pipeline in Europe
- In August 2024, AstraZeneca launched a global clinical trial program for its next-generation PARP inhibitor in cervical cancer, involving over 20 countries and 3,000 patients
- In March 2024, Pfizer expanded its cervical cancer collaboration with academic institutions in India for biomarker-based treatment approaches and real-world data collection
- In November 2023, Merck & Co. partnered with a leading diagnostics firm to co-develop companion diagnostics for stratifying cervical cancer patients based on PD-L1 expression
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.