Global Cervical Dilator Market
Market Size in USD Million
CAGR :
% 
 USD
67.59 Million
USD
84.43 Million
2024
2032
USD
67.59 Million
USD
84.43 Million
2024
2032
| 2025 –2032 | |
| USD 67.59 Million | |
| USD 84.43 Million | |
|
|
|
|
Cervical Dilator Market Size
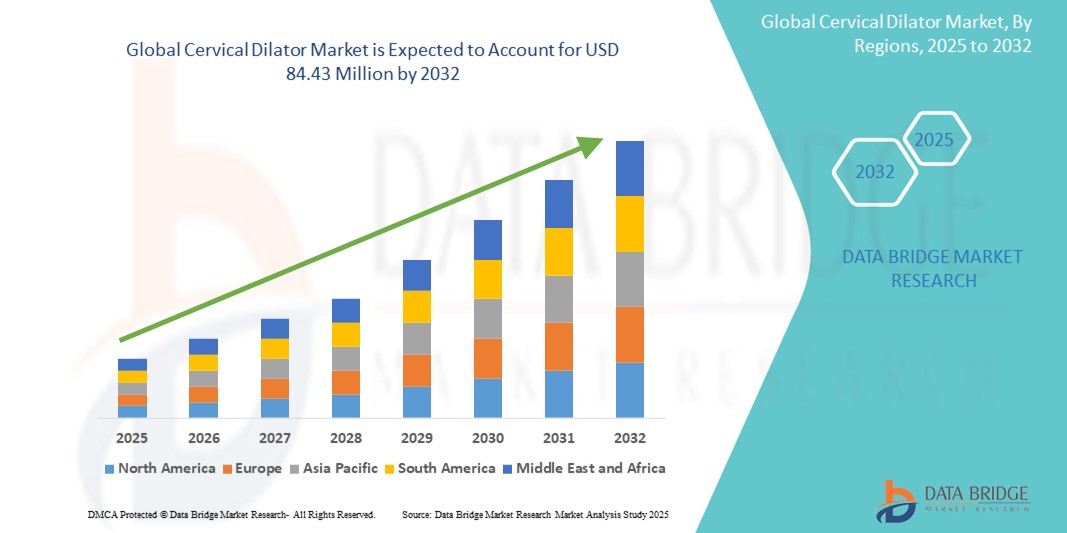
- The global cervical dilator market size was valued at USD 67.59 million in 2024 and is expected to reach USD 84.43 million by 2032, at a CAGR of 2.82% during the forecast period
- The market growth is primarily driven by the increasing prevalence of gynecological disorders, rising demand for minimally invasive surgical procedures, and advancements in dilation technologies that enhance patient safety and procedural efficiency
- Moreover, a growing number of diagnostic and therapeutic gynecological procedures, especially in emerging economies, is fostering demand for cervical dilators as essential tools in obstetric and gynecologic care. These trends are collectively strengthening market penetration and expanding the global footprint of cervical dilator solutions
Cervical Dilator Market Analysis
- Cervical dilators, essential medical devices used to gradually widen the cervix for diagnostic and therapeutic gynecological procedures, are increasingly crucial in obstetrics and gynecology due to their role in enhancing procedural safety and efficiency across both hospital and clinical settings
- The rising demand for cervical dilators is primarily driven by the increasing incidence of gynecological conditions, expanding awareness of women's health, and a growing preference for minimally invasive procedures that reduce patient discomfort and recovery time
- North America dominated the cervical dilator market with the largest revenue share of 39.5% in 2024, attributed to advanced healthcare infrastructure, favorable reimbursement policies, and the presence of major medical device manufacturers, with the U.S. exhibiting strong adoption due to a high volume of gynecological procedures and ongoing product innovation
- Asia-Pacific is projected to be the fastest growing region during the forecast period due to improving healthcare access, growing awareness of reproductive health, and rising investments in women's health services
- Double Ended segment dominated the cervical dilator market with a market share of 57.8% in 2024, driven by its versatility in performing multiple procedures and ability to provide efficient dilation with a single instrument
Report Scope and Cervical Dilator Market Segmentation
|
Attributes |
Cervical Dilator Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cervical Dilator Market Trends
“Technological Advancements in Minimally Invasive Gynecology”
- A key and accelerating trend in the global cervical dilator market is the shift towards technologically advanced, minimally invasive, and patient-friendly cervical dilation methods, which are transforming gynecological diagnostics and treatments
- For instance, osmotic dilators such as Dilapan-S are gaining popularity due to their gentle, controlled dilation and reduced risk of cervical trauma compared to traditional mechanical options. These devices absorb moisture and expand gradually, providing a safer, more comfortable alternative for both patients and practitioners
- Further innovations include cervical dilators designed for single-use with antimicrobial materials, aimed at reducing infection risk and ensuring procedural hygiene. Some models now incorporate ergonomic designs and softer materials to enhance patient comfort during use
- The integration of pre-sterilized, disposable dilators in outpatient gynecology and fertility clinics is increasing, supporting faster turnover rates and compliance with stricter healthcare standards
- This trend reflects a growing demand for cervical dilators that minimize patient discomfort, reduce procedural time, and align with evolving standards in modern gynecological care.
- Leading players such as CooperSurgical and Cook Medical are investing in product development and R&D to enhance efficacy and user experience in cervical dilation procedures
Cervical Dilator Market Dynamics
Driver
“Rising Demand Due to Growing Gynecological Procedures and Women's Health Awareness”
- The increasing prevalence of conditions such as cervical stenosis, infertility, and menstrual disorders, alongside heightened awareness of women’s health, is significantly driving demand for cervical dilators across hospitals and gynecology clinics
- For instance, the growing number of intrauterine device (IUD) placements, hysteroscopies, and fertility treatments globally necessitates reliable and efficient cervical dilation, positioning these tools as essential components in routine gynecological care
- Expanding access to healthcare in emerging markets and government initiatives promoting maternal and reproductive health are further accelerating the adoption of cervical dilators
- Moreover, the trend toward outpatient procedures and ambulatory surgical centers is boosting demand for portable, single-use, and easily sterilizable dilation devices
- The shift toward personalized and preventive gynecologic care, along with increasing investments in women’s health infrastructure and services, is contributing to the steady growth of the cervical dilator market
Restraint/Challenge
“Risk of Cervical Injury and Regulatory Compliance Constraints”
- Despite advancements, concerns over potential cervical trauma, pain, or uterine perforation during dilation procedures remain a significant challenge, particularly with traditional rigid dilators
- These risks may deter some practitioners from adopting mechanical options, especially for sensitive cases such as infertility or early pregnancy interventions
- In addition, stringent regulatory approvals and quality compliance requirements for medical devices can delay the entry of innovative cervical dilators into the market. Regulatory bodies such as the FDA and CE impose rigorous standards on safety, biocompatibility, and efficacy, increasing time-to-market and R&D costs for manufacturers
- Furthermore, the limited availability of skilled personnel trained in advanced gynecological techniques in lower-income regions restricts widespread adoption
- Overcoming these challenges requires continuous innovation in safer materials, better clinician training, and adherence to global quality standards to improve patient outcomes and boost practitioner confidence in using cervical dilators
Cervical Dilator Market Scope
The market is segmented on the basis of product type, type, material type, application, and end user.
- By Product Type
On the basis of product type, the cervical dilator market is segmented into double ended and single ended. The double ended segment dominated the market with the largest market revenue share of 57.8% in 2024, driven by its dual-functionality that enables efficient dilation with a single instrument, reducing procedural time and instrument costs. These dilators are widely used in high-volume gynecological practices for their clinical flexibility and convenience.
The single ended segment is expected to witness steady adoption during forecast period, particularly in low-risk procedures or settings with limited sterilization infrastructure, offering simplicity and ease of use.
- By Type
On the basis of type, the cervical dilator market is segmented into reusable and disposable. The reusable segment held the largest market revenue share of 61.4% in 2024, due to their durability and cost-effectiveness over repeated procedures, making them the preferred choice in hospitals and surgical centers.
The disposable segment is anticipated to witness the fastest growth from 2025 to 2032, driven by growing emphasis on infection control, patient safety, and procedural efficiency, especially in ambulatory and outpatient settings.
- By Material Type
On the basis of material type, the cervical dilator market is segmented into metal, plastic, resin type, and osmotic. The metal segment dominated the market with the largest market revenue share of 46.8% in 2024, owing to its high strength, precision, and reusability in complex gynecological procedures.
The osmotic segment is expected to grow at the fastest CAGR during the forecast period, fueled by increasing demand for non-invasive, gradual dilation methods that enhance patient comfort and safety, particularly in labor induction and fertility applications.
- By Application
On the basis of application, the cervical dilator market is segmented into IUD placement and removal, cervical stenosis treatment, fertility treatment, parturition, and others. The IUD placement and removal segment dominated the market with the largest market revenue share of 33.6% in 2024, supported by the global increase in IUD usage and the demand for precise and safe cervical access during device insertion and removal.
The fertility treatment segment is anticipated to experience strong growth from 2025 to 2032, as assisted reproductive procedures become more widespread and cervical dilation is increasingly required as a preparatory step.
- By End User
On the basis of end user, the cervical dilator market is segmented into hospitals, gynecology clinics, and ambulatory surgical centers. The hospital segment dominated the market with the largest market revenue share of 49.7% in 2024, driven by high procedure volumes, access to specialized instruments, and skilled healthcare professionals capable of performing advanced gynecological treatments.
The gynecology clinics segment is projected to grow steadily during forecast period, due to increased awareness of reproductive health and rising demand for routine outpatient gynecological care. Ambulatory surgical centers are also expanding due to cost-effective services and the rising preference for minimally invasive, same-day procedures.
Cervical Dilator Market Regional Analysis
- North America dominated the cervical dilator market with the largest revenue share of 39.5% in 2024, attributed to advanced healthcare infrastructure, favorable reimbursement policies, and the presence of major medical device manufacturers
- Consumers and healthcare providers in the region prioritize advanced, safe, and minimally invasive dilation solutions, leading to widespread adoption of both mechanical and osmotic dilators across hospitals and specialized clinics
- This dominance is further supported by strong government healthcare spending, favorable reimbursement frameworks, and active participation by leading market players in research and innovation, positioning North America as a key hub for cervical dilator adoption and technological advancement
U.S. Cervical Dilator Market Insight
The U.S. cervical dilator market captured the largest revenue share of 80.2% in 2024 within North America, driven by the high volume of gynecological procedures, early adoption of advanced medical technologies, and a robust healthcare infrastructure. Rising awareness of women’s health and the increasing use of IUDs, hysteroscopies, and fertility treatments further stimulate demand. Moreover, the presence of leading medical device manufacturers and strong investment in research and clinical innovation continue to accelerate market growth in the U.S.
Europe Cervical Dilator Market Insight
The Europe cervical dilator market is projected to grow at a steady CAGR throughout the forecast period, supported by increasing awareness of reproductive health, the rising number of fertility procedures, and strong public healthcare systems. The demand for minimally invasive gynecological tools is growing across countries such as Germany, France, and Italy, with healthcare providers integrating both reusable and disposable dilation devices in clinical practice. Furthermore, strict regulatory standards and growing investments in women-centric medical technologies are driving adoption.
U.K. Cervical Dilator Market Insight
The U.K. cervical dilator market is expected to expand at a significant CAGR during the forecast period, fueled by the rising prevalence of cervical conditions, greater awareness around early diagnosis, and a strong emphasis on women’s health initiatives. The country’s well-organized healthcare system, combined with increasing fertility and preventive screening procedures, supports the growing need for safe and effective cervical dilation tools. Moreover, hospital and outpatient centers continue to incorporate advanced devices to improve patient outcomes and procedural efficiency.
Germany Cervical Dilator Market Insight
The Germany cervical dilator market is anticipated to grow steadily, driven by its advanced medical infrastructure, high standard of gynecological care, and strong demand for technologically sophisticated and eco-conscious dilation tools. Hospitals and clinics in Germany are increasingly adopting osmotic and disposable dilators to align with best practices in patient safety and hygiene. Furthermore, the nation’s focus on precision medicine and innovation is encouraging greater integration of improved cervical dilation techniques in fertility and diagnostic procedures.
Asia-Pacific Cervical Dilator Market Insight
The Asia-Pacific cervical dilator market is expected to grow at the fastest CAGR of 23.2% during the forecast period of 2025 to 2032, propelled by rising healthcare awareness, increasing gynecological disease burden, and improved access to reproductive healthcare. Countries such as China, India, and Japan are experiencing rapid healthcare infrastructure development and growing acceptance of outpatient gynecological procedures. Government-led programs promoting women’s health and expanding use of both affordable and advanced dilation tools are accelerating regional growth.
Japan Cervical Dilator Market Insight
The Japan cervical dilator market is advancing due to the country’s aging population, rising need for gynecological screenings, and focus on technological innovation in medical procedures. Hospitals and fertility centers in Japan are increasingly adopting safer, minimally invasive cervical dilation tools, particularly osmotic dilators, to enhance patient comfort and procedural outcomes. Furthermore, the integration of modern diagnostic equipment in women’s health clinics supports market expansion.
India Cervical Dilator Market Insight
The India cervical dilator market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by rising urbanization, increasing healthcare investments, and a growing emphasis on reproductive and maternal health. India is witnessing a surge in gynecology-focused clinics and fertility centers, where cervical dilators are essential tools. The availability of cost-effective products, government support for women’s health programs, and a large target population contribute significantly to the country’s market leadership in the region
Cervical Dilator Market Share
The cervical dilator industry is primarily led by well-established companies, including:
- CooperSurgical, Inc. (U.S.)
- MedGyn Products, Inc. (U.S.)
- Cook (U.S.)
- DILAS GmbH (Germany)
- Pregna International Ltd. (India)
- Wallach Surgical Devices (U.S.)
- Surgiwear Ltd. (India)
- Medline Industries LP (U.S.)
- Smiths Medical ASD, Inc. (U.S.)
- Sklar Surgical Instruments (U.S.)
- Gynex Corporation (U.S.)
- Integra LifeSciences Corporation (U.S.)
- Purple Surgical UK Ltd. (U.K.)
- Hospiline Equipments Pvt. Ltd. (India)
- Jiangsu Rixin Medical Equipment Co., Ltd. (China)
- PAULDRACH Medical GmbH & Co. KG (Germany)
- Kash Surgical (India)
- Shanghai Medical Instruments (Group) Ltd. Corp. (China)
- Pelican Feminine Healthcare Ltd. (U.K.)
- Amaryllis Healthcare (India)
What are the Recent Developments in Global Cervical Dilator Market?
- In April 2023, CooperSurgical Inc. launched an updated version of its Cervical Ripening Balloon, featuring enhanced patient comfort and improved safety mechanisms for labor induction. This innovation aims to provide a more effective alternative to pharmacological methods by offering a mechanical dilation solution that aligns with evidence-based obstetric practices. The development reinforces CooperSurgical’s ongoing commitment to advancing women's health through clinically reliable and patient-centered devices
- In March 2023, MedGyn Products, Inc. announced the expansion of its disposable cervical dilator line, introducing single-use options in multiple sizes to meet varying clinical needs. These dilators are pre-sterilized and designed for one-time use, addressing infection control concerns in outpatient gynecology and fertility clinics. This product expansion reflects the industry’s shift toward safer, more hygienic solutions and supports MedGyn’s strategy to offer high-quality, accessible women’s health tools worldwide
- In February 2023, Wallach Surgical Devices, a Trudell Medical company, received regulatory approval in key European markets for its next-generation osmotic cervical dilators. These devices are designed to gently and gradually dilate the cervix, offering a non-invasive option for diagnostic and therapeutic procedures. This milestone not only enables broader global reach for Wallach’s products but also highlights the growing demand for patient-friendly, non-pharmacological dilation solutions
- In January 2023, Cook Medical announced the launch of its NEW Flexi-Tip Cervical Dilator Set, featuring a flexible, tapered design to minimize patient discomfort and reduce the risk of cervical trauma. The innovation is aimed at enhancing the safety and ease of IUD insertions, biopsies, and other gynecologic procedures. This new offering exemplifies Cook Medical’s focus on clinical performance and patient safety in reproductive health solutions
- In January 2023, Pregna International Ltd., a leading manufacturer of gynecological devices based in India, partnered with multiple public health organizations to supply cost-effective mechanical cervical dilators to rural health centers across Southeast Asia and Africa. This initiative seeks to improve access to essential reproductive health tools in underserved regions, reinforcing Pregna’s mission to bridge healthcare disparities through affordable and scalable medical technologies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CERVICAL DILATOR MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CERVICAL DILATOR MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CERVICAL DILATOR MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 GLOBAL CERVICAL DILATOR MARKET, BY TYPE
17.1 OVERVIEW
17.2 HEGER CERVICAL DILATOR
17.2.1 BY PRODUCT
17.2.1.1. SINGLE ENDED
17.2.1.2. DOUBLE ENDED
17.2.2 BY SIZE
17.2.2.1. BELOW 11MM/12MM
17.2.2.2. 11MM/12MM- 15/16MM
17.2.2.3. ABOVE 15MM/16MM
17.2.3 BY SHAPE
17.2.3.1. ROUND HOLLOW
17.2.3.2. SLIGHTLY CURVED
17.2.3.3. NON-FLEXIBLE
17.3 PRATT CERVICAL DILATOR
17.3.1 BY SIZE
17.3.1.1. BELOW 25/27 FR
17.3.1.2. 25/27FR -49/51 FR
17.3.1.3. ABOVE 49/51 FR
17.4 HANK CERVICAL DILATOR
17.4.1 BY SIZE
17.4.1.1. BELOW 13/14 FR
17.4.1.2. 13/14 FR -17/18 FR
17.4.1.3. ABOVE 17/18 FR
17.5 BALLON CERVICAL DILATOR
17.5.1 BY SIZE
17.5.1.1. 5MM
17.5.1.2. 7MM
17.5.1.3. 9MM
17.6 UTERINE ENDOCERVICAL CURETTE
17.6.1 BY TIP TYPE
17.6.1.1. SQUARE TIP
17.6.1.2. ROUND TIP
17.6.2 BY TYPE
17.6.2.1. TOWNSEND METAL UTERINE ENDOCERVICAL CURETTE
17.6.2.2. SPIRAL ENDOCERVICAL CURETTE
17.6.2.3. OTHERS
17.7 OSMOTIC CERVICAL DILATOR
17.8 OTHERS
18 GLOBAL CERVICAL DILATOR MARKET, BY PRODUCT MATERIAL
18.1 OVERVIEW
18.2 METAL CERVICAL DILATORS
18.2.1 STAINLESS STEEL
18.2.2 TITANIUM
18.2.3 OTHERS
18.3 NON-METAL CERVICAL DILATORS
18.3.1 PLASTIC AND RESINS
18.3.2 RUBBER
18.3.3 OSMOTIC (HYDROGEL)
18.3.4 OTHERS
19 GLOBAL CERVICAL DILATOR MARKET, BY USABILITY
19.1 OVERVIEW
19.2 REUSABLE
19.3 DISPOSABLE
20 GLOBAL CERVICAL DILATOR MARKET, BY STERILITY
20.1 OVERVIEW
20.2 STERILE
20.3 NON STERILE
21 GLOBAL CERVICAL DILATOR MARKET,BY SIZE
21.1 OVERVIEW
21.2 SMALL
21.3 MEDIUM
21.4 LARGE
22 GLOBAL CERVICAL DILATOR MARKET, BY APPLICATION
22.1 OVERVIEW
22.2 TREATMENT
22.2.1 INDUCTION OF LABOR
22.2.1.1. METAL CERVICAL DILATORS
22.2.1.2. NON-METAL CERVICAL DILATORS
22.2.2 PREGNANCY TERMINATION
22.2.2.1. METAL CERVICAL DILATORS
22.2.2.2. NON-METAL CERVICAL DILATORS
22.2.3 UTERINE CURETTAGE
22.2.3.1. METAL CERVICAL DILATORS
22.2.3.2. NON-METAL CERVICAL DILATORS
22.2.4 PLACEMENT AND REMOVAL OF INTRAUTERINE DEVICES
22.2.4.1. METAL CERVICAL DILATORS
22.2.4.2. NON-METAL CERVICAL DILATORS
22.2.5 OTHERS
22.3 DIAGNOSTICS
22.3.1 CERVICAL CANCER DIAGNOSIS
22.3.1.1. METAL CERVICAL DILATORS
22.3.1.2. NON-METAL CERVICAL DILATORS
22.3.2 OTHERS
23 GLOBAL CERVICAL DILATOR MARKET, BY END USER
23.1 OVERVIEW
23.2 HOSPITALS
23.3 SPECIALTY CLINICS
23.4 AMBULATORY SURGICAL CENTERS
23.5 TRAUMA CENTERS
23.6 DIAGNOSTIC CENTERS
23.7 ACADEMIC AND RESEARCH INSTITUTES
23.8 OTHERS
24 GLOBAL CERVICAL DILATOR MARKET, BY DISTRIBUTION CHANNEL
24.1 OVERVIEW
24.2 DIRECT TENDERS
24.3 RETAIL SALES
24.3.1 HOSPITAL PHARMACIES
24.3.2 DRUG STORES
24.3.3 ONLINE PHARMACIES
24.3.4 OTHERS
24.4 OTHERS
25 GLOBAL CERVICAL DILATOR MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.2 COMPANY SHARE ANALYSIS: EUROPE
25.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.4 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
25.5 COMPANY SHARE ANALYSIS: SOUTH AMERICA
25.6 MERGERS & ACQUISITIONS
25.7 NEW PRODUCT DEVELOPMENT & APPROVALS
25.8 EXPANSIONS
25.9 REGULATORY CHANGES
25.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL CERVICAL DILATOR MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL CERVICAL DILATOR MARKET, BY REGION
GLOBAL CERVICAL DILATOR MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
27.1 NORTH AMERICA
27.1.1 U.S.
27.1.2 CANADA
27.1.3 MEXICO
27.2 EUROPE
27.2.1 GERMANY
27.2.2 FRANCE
27.2.3 U.K.
27.2.4 HUNGARY
27.2.5 LITHUANIA
27.2.6 AUSTRIA
27.2.7 IRELAND
27.2.8 NORWAY
27.2.9 POLAND
27.2.10 ITALY
27.2.11 SPAIN
27.2.12 RUSSIA
27.2.13 TURKEY
27.2.14 NETHERLANDS
27.2.15 SWITZERLAND
27.2.16 REST OF EUROPE
27.3 ASIA-PACIFIC
27.3.1 JAPAN
27.3.2 CHINA
27.3.3 SOUTH KOREA
27.3.4 INDIA
27.3.5 AUSTRALIA
27.3.6 SINGAPORE
27.3.7 THAILAND
27.3.8 MALAYSIA
27.3.9 INDONESIA
27.3.10 PHILIPPINES
27.3.11 VIETNAM
27.3.12 REST OF ASIA-PACIFIC
27.4 SOUTH AMERICA
27.4.1 BRAZIL
27.4.2 ARGENTINA
27.4.3 PERU
27.4.4 COLOMBIA
27.4.5 VENEZUELA
27.4.6 REST OF SOUTH AMERICA
27.5 MIDDLE EAST AND AFRICA
27.5.1 SOUTH AFRICA
27.5.2 SAUDI ARABIA
27.5.3 UAE
27.5.4 EGYPT
27.5.5 KUWAIT
27.5.6 ISRAEL
27.5.7 REST OF MIDDLE EAST AND AFRICA
27.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
28 GLOBAL CERVICAL DILATOR MARKET, COMPANY PROFILE
28.1 INTEGRA LIFESCIENCES
28.1.1 COMPANY OVERVIEW
28.1.2 REVENUE ANALYSIS
28.1.3 GEOGRAPHIC PRESENCE
28.1.4 PRODUCT PORTFOLIO
28.1.5 RECENT DEVELOPMENTS
28.2 COOPER SURGICAL
28.2.1 COMPANY OVERVIEW
28.2.2 REVENUE ANALYSIS
28.2.3 GEOGRAPHIC PRESENCE
28.2.4 PRODUCT PORTFOLIO
28.2.5 RECENT DEVELOPMENTS
28.3 GERMEDUSA
28.3.1 COMPANY OVERVIEW
28.3.2 REVENUE ANALYSIS
28.3.3 GEOGRAPHIC PRESENCE
28.3.4 PRODUCT PORTFOLIO
28.3.5 RECENT DEVELOPMENTS
28.4 MEDGYN PRODUCTS
28.4.1 COMPANY OVERVIEW
28.4.2 REVENUE ANALYSIS
28.4.3 GEOGRAPHIC PRESENCE
28.4.4 PRODUCT PORTFOLIO
28.4.5 RECENT DEVELOPMENTS
28.5 MEDICEM, INC.
28.5.1 COMPANY OVERVIEW
28.5.2 REVENUE ANALYSIS
28.5.3 GEOGRAPHIC PRESENCE
28.5.4 PRODUCT PORTFOLIO
28.5.5 RECENT DEVELOPMENTS
28.6 SKLAR SURGICAL INSTRUMENTS
28.6.1 COMPANY OVERVIEW
28.6.2 REVENUE ANALYSIS
28.6.3 GEOGRAPHIC PRESENCE
28.6.4 PRODUCT PORTFOLIO
28.6.5 RECENT DEVELOPMENTS
28.7 NOVO SURGICAL INC.
28.7.1 COMPANY OVERVIEW
28.7.2 REVENUE ANALYSIS
28.7.3 GEOGRAPHIC PRESENCE
28.7.4 PRODUCT PORTFOLIO
28.7.5 RECENT DEVELOPMENTS
28.8 HOLOGIC, INC
28.8.1 COMPANY OVERVIEW
28.8.2 REVENUE ANALYSIS
28.8.3 GEOGRAPHIC PRESENCE
28.8.4 PRODUCT PORTFOLIO
28.8.5 RECENT DEVELOPMENTS
28.9 PANPAC MEDICAL CORP.
28.9.1 COMPANY OVERVIEW
28.9.2 REVENUE ANALYSIS
28.9.3 GEOGRAPHIC PRESENCE
28.9.4 PRODUCT PORTFOLIO
28.9.5 RECENT DEVELOPMENTS
28.1 MARINA MEDICAL INC.
28.10.1 COMPANY OVERVIEW
28.10.2 REVENUE ANALYSIS
28.10.3 GEOGRAPHIC PRESENCE
28.10.4 PRODUCT PORTFOLIO
28.10.5 RECENT DEVELOPMENTS
28.11 MEDLINE INDUSTRIES, LP.
28.11.1 COMPANY OVERVIEW
28.11.2 REVENUE ANALYSIS
28.11.3 GEOGRAPHIC PRESENCE
28.11.4 PRODUCT PORTFOLIO
28.11.5 RECENT DEVELOPMENTS
28.12 AMBLER SURGICAL
28.12.1 COMPANY OVERVIEW
28.12.2 REVENUE ANALYSIS
28.12.3 GEOGRAPHIC PRESENCE
28.12.4 PRODUCT PORTFOLIO
28.12.5 RECENT DEVELOPMENTS
28.13 SURTEX INSTRUMENTS LIMITED
28.13.1 COMPANY OVERVIEW
28.13.2 REVENUE ANALYSIS
28.13.3 GEOGRAPHIC PRESENCE
28.13.4 PRODUCT PORTFOLIO
28.13.5 RECENT DEVELOPMENTS
28.14 ADVIN HEALTH CARE
28.14.1 COMPANY OVERVIEW
28.14.2 REVENUE ANALYSIS
28.14.3 GEOGRAPHIC PRESENCE
28.14.4 PRODUCT PORTFOLIO
28.14.5 RECENT DEVELOPMENTS
28.15 STERIS
28.15.1 COMPANY OVERVIEW
28.15.2 REVENUE ANALYSIS
28.15.3 GEOGRAPHIC PRESENCE
28.15.4 PRODUCT PORTFOLIO
28.15.5 RECENT DEVELOPMENTS
28.16 SURGICAL HOLDINGS.
28.16.1 COMPANY OVERVIEW
28.16.2 REVENUE ANALYSIS
28.16.3 GEOGRAPHIC PRESENCE
28.16.4 PRODUCT PORTFOLIO
28.16.5 RECENT DEVELOPMENTS
28.17 GTIMD CATHETER SOLUTIONS
28.17.1 COMPANY OVERVIEW
28.17.2 REVENUE ANALYSIS
28.17.3 GEOGRAPHIC PRESENCE
28.17.4 PRODUCT PORTFOLIO
28.17.5 RECENT DEVELOPMENTS
28.18 COOK
28.18.1 COMPANY OVERVIEW
28.18.2 REVENUE ANALYSIS
28.18.3 GEOGRAPHIC PRESENCE
28.18.4 PRODUCT PORTFOLIO
28.18.5 RECENT DEVELOPMENTS
28.19 SHANGHAI MICROPORT MEDICAL (GROUP) CO., LTD.
28.19.1 COMPANY OVERVIEW
28.19.2 REVENUE ANALYSIS
28.19.3 GEOGRAPHIC PRESENCE
28.19.4 PRODUCT PORTFOLIO
28.19.5 RECENT DEVELOPMENTS
28.2 THOMAS MEDICAL.
28.20.1 COMPANY OVERVIEW
28.20.2 REVENUE ANALYSIS
28.20.3 GEOGRAPHIC PRESENCE
28.20.4 PRODUCT PORTFOLIO
28.20.5 RECENT DEVELOPMENTS
28.21 SURGIPRO, INC.
28.21.1 COMPANY OVERVIEW
28.21.2 REVENUE ANALYSIS
28.21.3 GEOGRAPHIC PRESENCE
28.21.4 PRODUCT PORTFOLIO
28.21.5 RECENT DEVELOPMENTS
28.21.6 COMPANY OVERVIEW
28.21.7 REVENUE ANALYSIS
28.21.8 GEOGRAPHIC PRESENCE
28.21.9 PRODUCT PORTFOLIO
28.21.10 RECENT DEVELOPMENTS
28.22 LABORIE
28.22.1 COMPANY OVERVIEW
28.22.2 REVENUE ANALYSIS
28.22.3 GEOGRAPHIC PRESENCE
28.22.4 PRODUCT PORTFOLIO
28.22.5 RECENT DEVELOPMENTS
28.23 MEDESIGN I.C. GMBH
28.23.1 COMPANY OVERVIEW
28.23.2 REVENUE ANALYSIS
28.23.3 GEOGRAPHIC PRESENCE
28.23.4 PRODUCT PORTFOLIO
28.23.5 RECENT DEVELOPMENTS
28.24 GYNEDIL.COM
28.24.1 COMPANY OVERVIEW
28.24.2 REVENUE ANALYSIS
28.24.3 GEOGRAPHIC PRESENCE
28.24.4 PRODUCT PORTFOLIO
28.24.5 RECENT DEVELOPMENTS
28.25 TELEFLEX INCORPORATED
28.25.1 COMPANY OVERVIEW
28.25.2 REVENUE ANALYSIS
28.25.3 GEOGRAPHIC PRESENCE
28.25.4 PRODUCT PORTFOLIO
28.25.5 RECENT DEVELOPMENTS
28.26 GYNEX
28.26.1 COMPANY OVERVIEW
28.26.2 REVENUE ANALYSIS
28.26.3 GEOGRAPHIC PRESENCE
28.26.4 PRODUCT PORTFOLIO
28.26.5 RECENT DEVELOPMENTS
29 RELATED REPORTS
30 CONCLUSION
31 QUESTIONNAIRE
32 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.