Global Chimeric Antigen Receptor T Car T Cells Market
Market Size in USD Billion
CAGR :
% 
 USD
5.36 Billion
USD
78.26 Billion
2024
2032
USD
5.36 Billion
USD
78.26 Billion
2024
2032
| 2025 –2032 | |
| USD 5.36 Billion | |
| USD 78.26 Billion | |
|
|
|
|
Chimeric Antigen Receptor T (CAR-T) Cells Market Analysis
In recent years, the chimeric antigen receptor (car) t-cell treatment sector has had exceptional growth, expected to continue in the coming years. Rising investments in research and development activities, the entry of new players, product innovation, technological breakthroughs, effective resource allocation, and growing competition among business rivals to expand its regional and customer base contribute to the industry's growth.
Chimeric Antigen Receptor T (CAR-T) Cells Market Size
Global Chimeric Antigen Receptor T (CAR-T) cells market size was valued at USD 5.36 billion in 2024 and is projected to reach USD 78.26 billion by 2032, with a CAGR of 39.82% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Chimeric Antigen Receptor T (CAR-T) Cells Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Novartis AG (Switzerland), Pfizer Inc. (U.S), Gilead Sciences, Inc. (U.S), Autolus Therapeutics (U.K), CARsgen Therapeutics Co.Ltd. (U.K), Juno Therapeutics, Inc. (U.S), Sorrento Therapeutics, Inc. (U.S), Legend Biotech (U.S), Calyxt Inc. (France), Mustang Bio (U.S), bluebird bio, Inc. (U.S), CELGENE CORPORATION (U.S), Eureka Therapeutics Inc. (U.S), Avacta Life Sciences Ltd. (U.K) |
|
Market Opportunities |
|
Chimeric Antigen Receptor T (CAR-T) Cells Market Definition
CAR-T therapy is a type of treatment in which a patient's T cells, which are immune cells, are genetically engineered in the lab to kill cancer cells. T cells are collected from a patient's blood. T cells in the lab are given the gene for a specific receptor that binds to a specific protein on the patient's cancer cells. Chimeric antigen receptors are a special kind of receptor (CAR). CAR-T cells are mass-produced in the lab and then injected into the patient. CAR-T therapy is used to treat certain types of blood cancers and is also being studied for use in other cancers.
Chimeric Antigen Receptor T (CAR-T) Cells Market Dynamics
Drivers
- Increasing occurrences of cancer
In the forecast period of 2025-2032, rising levels of investment for the development of advanced and technical solutions and products, an increase in the number of cell therapy clinical studies, and the growth of the pharmaceutical industry will all help to accelerate the growth of the chimeric antigen receptor T (CAR-T) cells market. In the forecast period, increasing use of cells for the treatment of various hematologic and solid tumour types would enhance numerous prospects, resulting in the expansion of the chimeric antigen receptor T (CAR-T) cells market.
- Increasing number of patients
The rise in the frequency of cancer, the increase in the number of patients who have failed to respond to alternative treatments, and the rise in healthcare costs are all predicted to promote the growth of the chimeric antigen receptor (CAR)-T cell therapy market in the forecast period.
- Increasing approvals for Car-T cell therapy products
As people become more aware of this novel way of treating cancer, demand for auto T cell therapy products rises. As a result, leading market players are working on new products, which is driving the industry forward. The US Food and Drug Administration authorized Yescarta, a cell-based gene therapy, in October 2017 to treat adult patients with certain kinds of large B-cell lymphoma. Yescarta's clearance is a breakthrough in the field of CAR-T cell treatments for cancer patients.
- Increasing R&D therapies
By 2028, the global CAR T-cell treatment industry is estimated to reach $15 billion. CAR T-cell therapy providers are likely to benefit from increased attention on research and development medicines for cancers such as lung cancer, colorectal cancer, multiple myeloma cancer, and prostate cancer. In addition to cancer, researchers are verifying the function of therapy in a variety of other diseases, such as HIV, autoimmune disorders, infections, and others, which will drive the market's growth throughout the forecast period.
Opportunities
In addition, as people become more aware of efficiency, the market for chimeric antigen receptor (CAR)-T cell treatment is expected to develop in the coming years. However, the potential for cytokine release syndrome (CRS) and other neurological disorders as a result of cell therapy could provide further growth prospects for the chimeric antigen receptor (CAR)-T cell therapy industry in the near future.
Restraints/Challenges
In the predictable future, high treatment costs and negative effects associated with CAR T cell use would operate as a market restraint for the expansion of chimeric antigen receptor T (CAR-T) cells.
This chimeric antigen receptor T (CAR-T) cells market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the chimeric antigen receptor T (CAR-T) cells market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Chimeric Antigen Receptor T (CAR-T) Cells Market Scope
The chimeric antigen receptor T (CAR-T) cells market is segmented on the basis of target antigen, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Target Antigen
- CD19
- CD22
- Others
Application
- Acute Lymphoblastic Leukaemia
- Diffuse Large B-Cell Lymphoma
- Others
End User
- Hospitals
- Cancer Research Centres
- Clinics
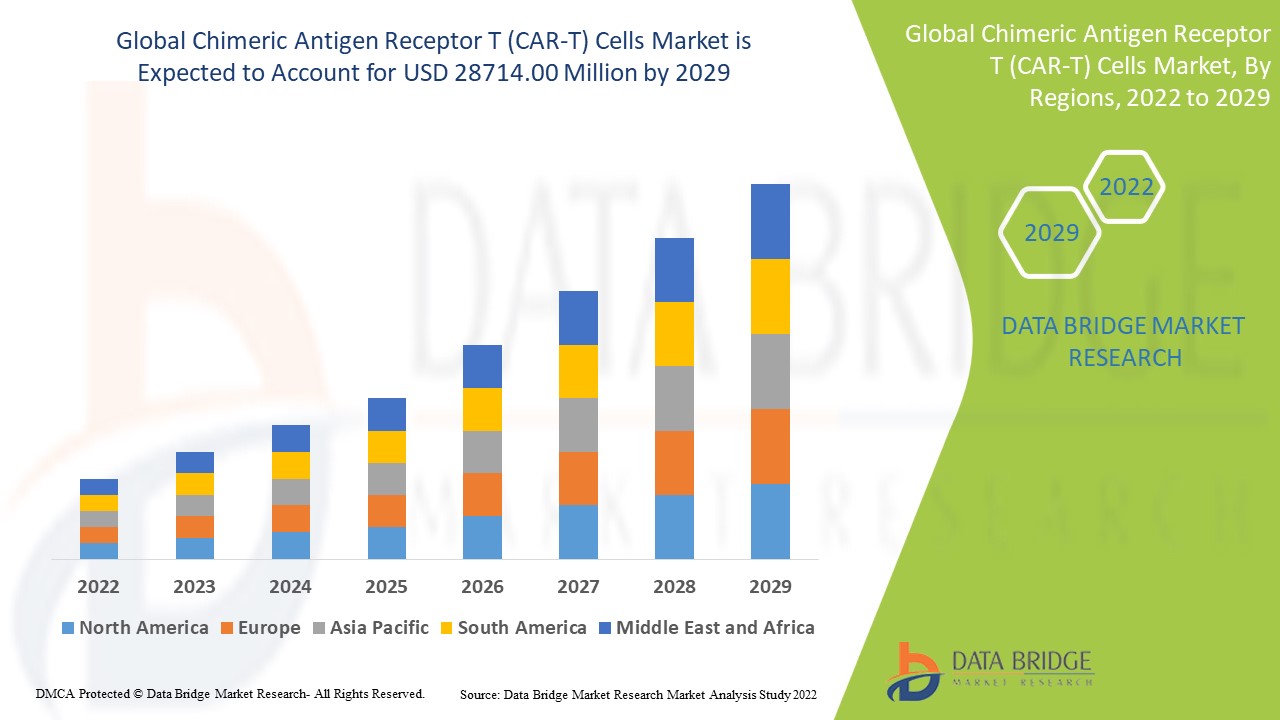
Chimeric Antigen Receptor T (CAR-T) Cells Market Regional Analysis
The chimeric antigen receptor T (CAR-T) cells market is analyzed and market size insights and trends are provided by country, target antigen, application and end-user as referenced above.
The countries covered in the chimeric antigen receptor T (CAR-T) cells market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominating the chimeric antigen receptor T (CAR-T) cells market due to the high prevalence of cancer.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2025 to 2032 due to the prevalence of better healthcare infrastructure along with growing disposable income of the people.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Chimeric Antigen Receptor T (CAR-T) Cells Market Share
The chimeric antigen receptor T (CAR-T) cells market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to chimeric antigen receptor T (CAR-T) cells market.
Chimeric Antigen Receptor T (CAR-T) Cells Market Leaders Operating in the Market Are:
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S)
- Gilead Sciences, Inc. (U.S)
- Autolus Therapeutics (U.K)
- CARsgen Therapeutics Co. Ltd. (U.K)
- Juno Therapeutics, Inc.(U.S)
- Sorrento Therapeutics, Inc. (U.S)
- Legend Biotech (U.S)
- Calyxt Inc. (France)
- Mustang Bio (U.S)
- bluebird bio, Inc. (U.S)
- CELGENE CORPORATION (U.S)
- Eureka Therapeutics Inc. (U.S)
- Avacta Life Sciences Ltd. (U.K)
Latest Developments in Chimeric Antigen Receptor T (CAR-T) Cells Market
- In February 2021, After getting FDA permission for commercialization in the United States, Bristol Myers Squibb marketed Breyanzi (lisocabtagene maraleucel). Breyanzi is a cell-based gene therapy used to treat certain kinds of big B-cell lymphoma in adults
- In July 2020, After getting FDA permission for commercialization in the United States, Kite Pharma, a Gilead Sciences subsidiary, introduced Tecartus. Tecartus (brexucabtagene autoleucel, formerly KTE-X19) is the first and only CAR T-cell therapy licenced for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.