Global Chordoma Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.98 Billion
USD
6.64 Billion
2024
2032
USD
3.98 Billion
USD
6.64 Billion
2024
2032
| 2025 –2032 | |
| USD 3.98 Billion | |
| USD 6.64 Billion | |
|
|
|
|
Chordoma Treatment Market Size
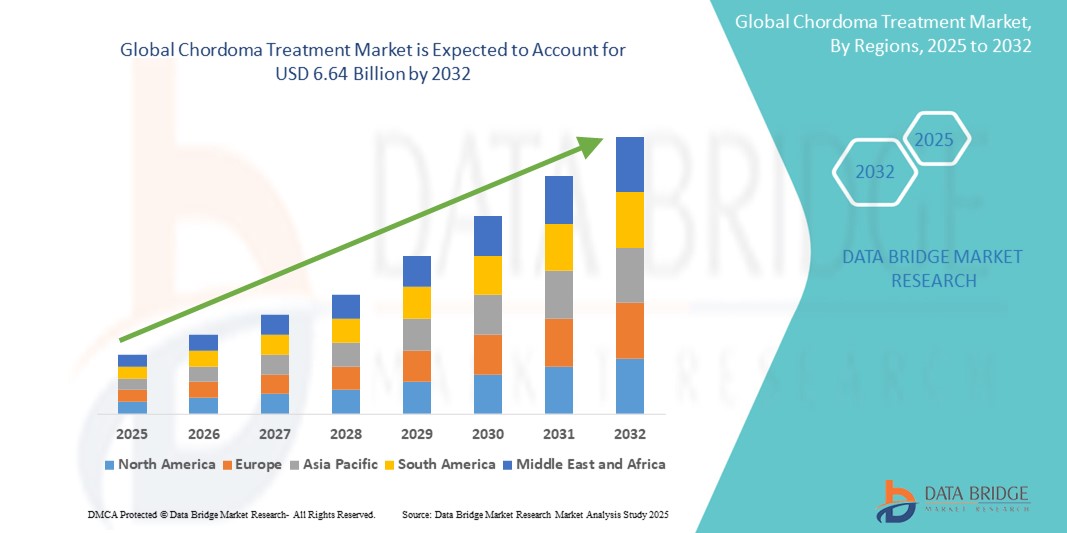
- The global chordoma treatment market size was valued at USD 3.98 billion in 2024 and is expected to reach USD 6.64 billion by 2032, at a CAGR of 6.60% during the forecast period
- The market growth is largely driven by increased awareness and improved diagnostic capabilities for rare cancers, alongside a growing emphasis on targeted therapy and personalized medicine in oncology
- Furthermore, the development of novel drugs, rising clinical trial activity, and expanded research funding for rare tumors are strengthening the treatment landscape. These converging factors are accelerating advancements in chordoma care, thereby significantly boosting the industry's growth
Chordoma Treatment Market Analysis
- Chordoma treatment involves a multidisciplinary approach including surgery, radiation therapy, and emerging targeted therapies aimed at managing this rare, slow-growing malignant bone tumor, most commonly found along the spine and skull base. Increasing clinical awareness and access to advanced imaging and diagnostic tools are enhancing early detection and treatment efficacy
- The escalating demand for chordoma treatment is primarily fueled by the growing prevalence of rare cancers, increased research focus on orphan diseases, and advancements in precision oncology that offer more effective and personalized treatment options
- North America dominated the chordoma treatment market with the largest revenue share of 41.8% in 2024, driven by a robust healthcare infrastructure, high healthcare spending, and the presence of leading research institutions conducting clinical trials and developing novel therapies, particularly in the U.S. where patient advocacy and rare disease funding play key roles
- Asia-Pacific is expected to be the fastest growing region in the chordoma treatment market during the forecast period due to increasing healthcare investments, improved access to specialized cancer care, and rising awareness of rare diseases
- The targeted therapy segment dominated the chordoma treatment market with a market share of 46.2% in 2024, propelled by the growing availability of molecular diagnostics and the effectiveness of precision treatments such as tyrosine kinase inhibitors in managing chordoma
Report Scope and Chordoma Treatment Market Segmentation
|
Attributes |
Chordoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Chordoma Treatment Market Trends
“Advancements in Targeted Therapy and Molecular Diagnostics”
- A significant and accelerating trend in the global chordoma treatment market is the increasing shift toward targeted therapy and molecular diagnostics, driven by advances in genomics and the understanding of chordoma’s biological mechanisms. These innovations are enabling more personalized, effective, and less invasive treatment strategies
- For instance, therapies targeting brachyury—a key transcription factor overexpressed in chordoma—are under active investigation. Clinical trials such as those exploring the efficacy of afatinib and other tyrosine kinase inhibitors (TKIs) represent efforts to create treatments tailored to chordoma’s molecular profile
- Molecular diagnostics, including next-generation sequencing (NGS), are becoming instrumental in identifying actionable mutations and stratifying patients based on their genetic profiles. This facilitates more precise treatment selection and helps in the design of adaptive clinical trials
- The integration of molecular data into clinical practice enables oncologists to offer personalized treatment regimens and monitor disease progression more accurately. This is transforming the chordoma care paradigm from generalized management to precision oncology
- Pharmaceutical and biotech companies are increasingly collaborating with research institutions to accelerate the development of biologics and small-molecule drugs targeting rare cancers. For instance, companies such as OncoFusion Therapeutics are focusing on fusion gene-driven cancers such as chordoma with innovative, mechanism-based therapies
- The growing demand for effective, minimally invasive treatment solutions is pushing the development of targeted therapeutics and advanced diagnostics across global markets, especially in regions with improving healthcare infrastructure and access to specialized cancer care
Chordoma Treatment Market Dynamics
Driver
“Rising Research Focus on Rare Cancers and Orphan Drug Development”
- The rising incidence of rare cancers, combined with increasing global research investments in orphan diseases, is a major driver of the chordoma treatment market
- For instance, in early 2024, the Chordoma Foundation partnered with international cancer research institutions to launch new preclinical and early-stage clinical trials focused on immunotherapy and gene-specific approaches for chordoma. Such collaborative initiatives are expected to accelerate drug development and expand treatment options
- Regulatory incentives such as orphan drug status, fast-track approvals, and market exclusivity are encouraging pharmaceutical companies to invest in developing therapies for low-prevalence conditions such as chordoma
- Moreover, increased advocacy and awareness campaigns led by patient support organizations are enhancing early diagnosis and facilitating patient enrollment in clinical trials, which is essential for generating real-world data and refining treatment strategies
- As medical professionals and institutions become more equipped to diagnose and manage rare cancers, chordoma is increasingly treated through specialized centers using a multidisciplinary approach, further driving demand for novel therapeutics
Restraint/Challenge
“Limited Treatment Options and Diagnostic Delay”
- One of the key challenges facing the chordoma treatment market is the limited availability of approved therapies and the often-delayed diagnosis due to the tumor’s rarity and nonspecific symptoms
- Misdiagnosis or delayed identification can result in disease progression and reduced treatment efficacy. The lack of chordoma-specific diagnostic guidelines in general practice settings contributes to this issue
- In addition, the current standard of care—often involving complex surgical resection combined with high-dose radiation—presents challenges such as potential morbidity, limited access to expertise, and high treatment costs, especially in low- and middle-income countries
- High development costs and small patient populations also make it difficult for pharmaceutical companies to achieve commercial viability without significant funding or incentives
- Overcoming these barriers will require increased investment in early diagnosis tools, broader access to genomic profiling, expansion of specialized treatment centers, and global collaboration to bring new therapies through the development pipeline and into clinical use
Chordoma Treatment Market Scope
The market is segmented on the basis of diagnosis, treatment type, and end user.
- By Diagnosis
On the basis of diagnosis, the chordoma treatment market is segmented into biopsy, imaging, and blood tests. The imaging segment dominated the market with the largest revenue share in 2024, attributed to its central role in the initial identification, staging, and treatment planning of chordoma. Advanced imaging techniques such as MRI and CT scans are essential for precise localization of tumors along the spine or skull base, offering critical anatomical detail for surgical and radiation therapy planning. The increasing use of image-guided technologies in oncology has also reinforced imaging’s dominance in this segment.
The biopsy segment is anticipated to witness the fastest growth rate from 2025 to 2032, owing to the rising reliance on histopathological confirmation and molecular profiling of tumors. Biopsy plays a vital role in the differentiation of chordoma from other bone neoplasms and in identifying genetic markers such as brachyury, which are crucial for targeted therapy decisions. As precision medicine becomes more central in oncology, the demand for accurate diagnostic biopsies is expected to grow substantially
- By Treatment Type
On the basis of treatment type, the chordoma treatment market is segmented into surgery, radiation therapy, chemotherapy, cryosurgery, and targeted therapy.
The targeted therapy segment dominated the market with the largest revenue of 46.2% share in 2024, driven by increasing adoption of precision medicine and growing recognition of chordoma's unique molecular targets such as brachyury. Targeted therapies, including tyrosine kinase inhibitors and monoclonal antibodies, offer a more focused mechanism of action, minimizing damage to surrounding tissues and improving treatment efficacy, especially in inoperable or recurrent cases. The rising number of clinical trials and regulatory approvals for rare cancer treatments have further reinforced the market position of this segment.
The surgery segment is expected to witness fastest growth from 2025 to 2032, supported by advancements in surgical techniques such as intraoperative navigation and robotic-assisted procedures. Although targeted therapies are gaining traction, surgery remains an essential part of the treatment pathway for localized chordoma, offering potential curative outcomes when complete resection is achieved. The integration of surgical care with postoperative therapies is also enhancing long-term patient outcomes.
- By End User
On the basis of end user, the chordoma treatment market is segmented into hospitals, clinics, and cancer care diagnostic centers. The hospitals segment led the market in 2024, supported by the availability of multidisciplinary teams, advanced diagnostic imaging infrastructure, and the capacity to conduct complex surgical procedures. Most chordoma treatments require hospitalization for surgical interventions or coordinated oncological care, making hospitals the primary end-use segment.
The cancer care diagnostic centers segment is expected to experience the fastest growth through 2032, fueled by increasing demand for outpatient cancer services, diagnostic imaging, and molecular testing. These centers offer specialized services with shorter waiting times and personalized care models, enhancing accessibility for chordoma patients. Growing investments in diagnostic technologies and targeted therapy support infrastructure at standalone centers are further accelerating segment growth.
Chordoma Treatment Market Regional Analysis
- North America dominated the chordoma treatment market with the largest revenue share of 41.8% in 2024, driven by a robust healthcare infrastructure, high healthcare spending, and the presence of leading research institutions conducting clinical trials and developing novel therapies, particularly in the United States where patient advocacy and rare disease funding play key roles
- Patients in the region benefit from early diagnosis and access to advanced treatment modalities including targeted therapies, precision diagnostics, and complex surgical interventions conducted by multidisciplinary teams
- This leadership is further supported by increased awareness of rare cancers, active patient advocacy groups, and a favorable regulatory environment promoting orphan drug development, positioning North America as the leading region for innovation and delivery in chordoma treatment
U.S. Chordoma Treatment Market Insight
The U.S. chordoma treatment market captured the largest revenue share of 80.5% in 2024 within North America, driven by advanced healthcare infrastructure, high awareness of rare cancers, and active research initiatives. The presence of specialized treatment centers, such as those affiliated with the Chordoma Foundation and National Cancer Institute-designated cancer centers, significantly supports diagnosis, therapy development, and patient access to clinical trials. Continued investment in precision medicine and orphan drug programs is further propelling market growth.
Europe Chordoma Treatment Market Insight
The Europe chordoma treatment market is projected to expand at a substantial CAGR throughout the forecast period, supported by strong government funding for rare disease research and a collaborative network of academic and clinical institutions. Rising adoption of personalized cancer treatment approaches and improvements in healthcare access across the region are contributing to increased diagnosis and advanced treatment of chordoma. Europe is also witnessing growing clinical trial activity, particularly in countries such as Germany and France.
U.K. Chordoma Treatment Market Insight
The U.K. chordoma treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by increasing public and private sector support for rare cancer treatment and research. National Health Service (NHS) initiatives for early cancer detection and access to novel therapies are enhancing chordoma patient outcomes. The presence of world-renowned oncology centers and research partnerships also strengthens the U.K.’s position in the global chordoma treatment landscape.
Germany Chordoma Treatment Market Insight
The Germany chordoma treatment market is expected to expand at a considerable CAGR during the forecast period, driven by a well-established healthcare system, cutting-edge cancer treatment facilities, and robust academic research. Government-backed rare disease initiatives and support for precision oncology programs are encouraging the development and adoption of innovative therapeutic approaches for chordoma. Germany’s centralized health data infrastructure further supports long-term patient monitoring and evidence-based care.
Asia-Pacific Chordoma Treatment Market Insight
The Asia-Pacific chordoma treatment market is poised to grow at the fastest CAGR of 23.6% during the forecast period of 2025 to 2032, propelled by increasing healthcare investments, growing awareness of rare diseases, and a rise in specialized oncology services in countries such as China, Japan, and India. Government efforts to expand healthcare coverage and promote rare disease diagnostics are enhancing access to chordoma treatment. In addition, expanding medical tourism and clinical trial capabilities are positioning APAC as a key emerging market.
Japan Chordoma Treatment Market Insight
The Japan chordoma treatment market is gaining momentum due to its strong emphasis on advanced cancer care, precision diagnostics, and technological innovation in healthcare. With a well-developed hospital infrastructure and government focus on rare disease management, Japan offers early access to advanced therapies. The integration of molecular diagnostics and national cancer registries supports the efficient tracking and management of chordoma cases.
India Chordoma Treatment Market Insight
The India chordoma treatment market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to expanding tertiary care infrastructure, rising cancer awareness, and increasing participation in international clinical trials. Government programs promoting rare disease awareness and diagnostics, combined with the growth of oncology centers in both public and private sectors, are driving demand. India’s large patient pool and affordability of care make it a key destination for clinical research and treatment innovation in chordoma.
Chordoma Treatment Market Share
The chordoma treatment industry is primarily led by well-established companies, including:
- AstraZeneca (U.K.)
- Amgen, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Lilly (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Pfizer, Inc. (U.S.)
- Sanofi (France)
- Debiopharm (Switzerland)
- Bayer AG (Germany)
- Johnson & Johnson Services, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Optivus Proton Therapy, Inc. (U.S.)
- Varian Medical Systems, Inc. (U.S.)
- Celldex Therapeutics (U.S.)
- BEBIG Medical. (Germany)
- GoDaddy Operating Company, LLC. (U.S.)
- Medivir AB (Sweden)
- Verdict Media Limited (U.K.)
What are the Recent Developments in Global Chordoma Treatment Market?
- In April 2023, Chimeric Therapeutics, an oncology-focused biotech company, announced the advancement of its CHM 2101 program, a novel CAR T-cell therapy targeting solid tumors, including chordoma. The therapy, which is based on chlorotoxin targeting IL13Rα2, entered preclinical development stages with promising anti-tumor activity. This progress highlights Chimeric’s commitment to developing next-generation immunotherapies for rare and hard-to-treat cancers, marking a significant step toward cell-based treatments in the chordoma landscape
- In March 2023, the Chordoma Foundation launched a global patient registry in collaboration with multiple clinical institutions, aimed at accelerating research and improving treatment protocols for chordoma patients. This initiative facilitates real-world data collection on patient outcomes, enabling more informed clinical decisions and fostering collaboration between researchers, healthcare providers, and pharmaceutical companies. The registry exemplifies the growing role of patient-centered data in guiding treatment innovations
- In February 2023, Novartis AG announced new clinical trial data on its investigational tyrosine kinase inhibitor for rare tumors, including chordoma. Preliminary results demonstrated favorable safety and efficacy profiles, reinforcing Novartis’ role in developing targeted treatments for niche oncology markets. This development emphasizes the potential of kinase inhibitors in managing advanced or inoperable chordoma cases
- In January 2023, OncoFusion Therapeutics, a biotech company specializing in fusion gene-driven cancers, initiated preclinical research on a new small molecule therapy specifically designed to target the brachyury gene, which is uniquely overexpressed in chordoma. The company’s work represents a promising direction for personalized therapy development and aligns with the growing demand for gene-targeted treatments in rare cancers
- In January 2023, Radiation Oncology Centers in the U.S. and Europe, including the Mayo Clinic and Heidelberg Ion-Beam Therapy Center, reported advancements in proton and carbon ion therapy protocols for chordoma. These cutting-edge radiation therapies offer higher precision and reduced side effects, making them increasingly preferred for tumors located near critical neurological structures. Such developments underscore the ongoing shift toward more effective and less invasive treatment options in the global chordoma care ecosystem
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.