Global Circulating Tumor Dna Ctdna Market
Market Size in USD Billion
CAGR :
% 
 USD
19.76 Billion
USD
69.40 Billion
2024
2032
USD
19.76 Billion
USD
69.40 Billion
2024
2032
| 2025 –2032 | |
| USD 19.76 Billion | |
| USD 69.40 Billion | |
|
|
|
|
Circulating Tumor DNA (ctDNA) Market Analysis
Nucleated cells lacking CD45 and expressing cytokeratin were classified as circulating tumor cells. The use of circulating tumor DNA diagnostics to identify cancer reduces the requirement for sample tumor tissue, such as tumor biopsy. Tumor DNA diagnostics in the blood are also used to guide tumor-specific treatment. This strategy aids the clinician in determining the most appropriate cancer treatment option.
Circulating Tumor DNA (ctDNA) Market Size
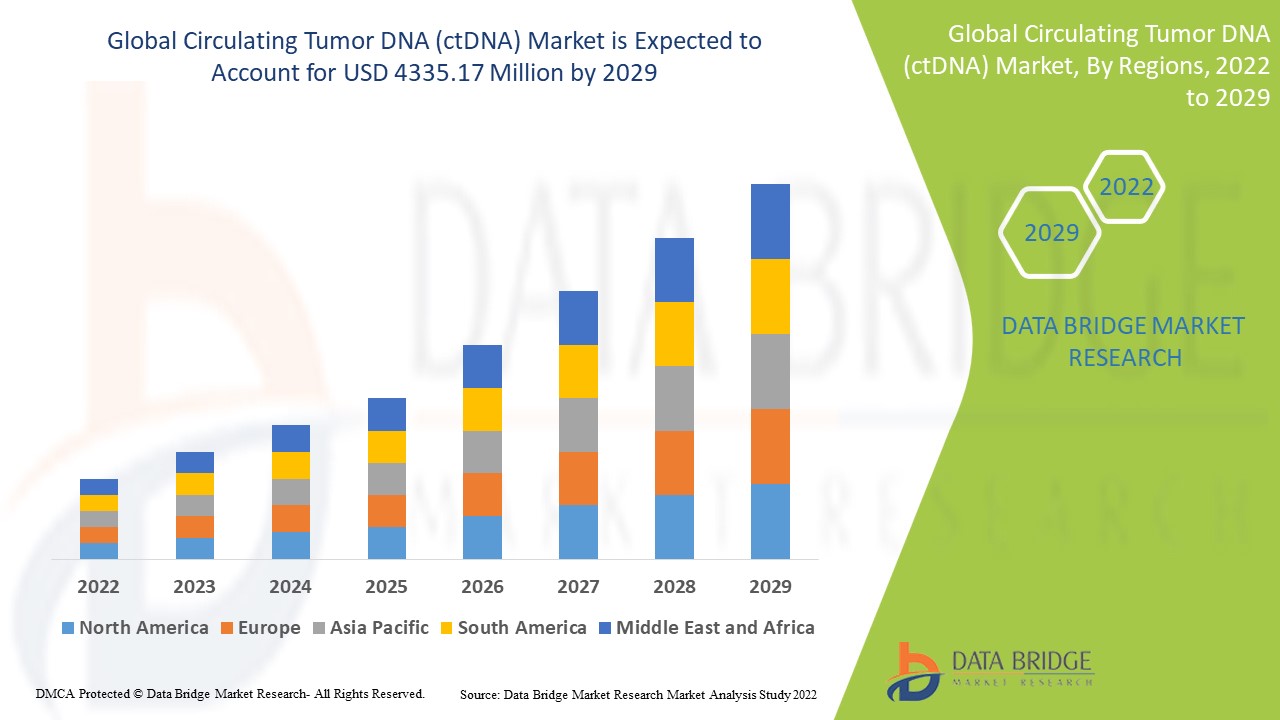
Global circulating tumor DNA (ctDNA) market size was valued at USD 19.76 billion in 2024 and is projected to reach USD 69.40 billion by 2032, with a CAGR of 17% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Circulating Tumor DNA (ctDNA) Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
GRAIL, Inc. (U.S), Guardant Health (U.S), Biodesix (U.S), Freenome Holdings, Inc. (U.S), LungLife AI, Inc. (U.S), Inivata Ltd (U.K), Personal Genome Diagnostics Inc. (U.S), CellMax Life (U.S), Vermillion Incorporated (U.S), Genomic Health Inc. (U.S), Foundation Medicine, Inc. (U.S), Biocept, Inc. (U.S), Myriad Genetics, Inc. (U.S), OncoCyte (U.S), Veracyte, Inc. (U.S) |
|
Market Opportunities |
|
Circulating Tumor DNA (ctDNA) Market Definition
Circulating tumor DNA (ctDNA) is a type of DNA produced by malignant and tumor cells that spread throughout the body. This occurs when a tumor grows and cells die, only to be replaced by a new one, releasing the dead cells into the bloodstream. This DNA are commonly found in leiomyosarcoma patients. Urine, saliva, stool, and cerebrospinal fluid all contain this DNA.
Circulating Tumor DNA (ctDNA) Market Dynamics
Drivers
- Increasing funding by the government for the development of test kits
The circulating tumor DNA (ctDNA) market is expected to grow during the projected period due to the rising demand for cancer diagnoses. Improved government funding is also helping the market to grow.
- Disease analysis and tracking
Standard tissue biopsy DNA, which only examines a small portion of the tumor, ctDNA provides an impartial picture of all mutations throughout the patient's overall tumor burden, thus lifting the growth of the circulating tumor DNA (ctDNA) market.
Moreover, the rising cases of leiomyosarcoma worldwide, affordable price of this method and increasing cases of cancer worldwide are also driving the market growth.
Opportunities
Additionally, ctDNA testing can be utilized with other multi-omic biomarkers to improve early detection. Early detection proteins (such as CA-125), epigenetic markers, circulating tumor RNA, nucleosomes, exosomes, and related immunological markers, for instance, may all be included in tests. Many firms are presently competing to create a commercially viable ctDNA-based early cancer detection tool. Although some challenges (such as early-stage disease assay accuracy, high implementation costs, confounding from clonal hematopoiesis, and a lack of clinical utility studies) must be overcome before ctDNA assays can be used in healthcare, they have a lot of promise as an early cancer screening test.
Restraints/Challenges
Shortage of skilled and trained professionals and problems related to NGS implementation are the factors that restrain the market growth. Rare 'events' - isolation is technically difficult. Profiling may be more expensive if blood background profiling is required, Single-cell/low cell number sequencing problematic (heterogeneity seen could be biological or technical bias), sampling bias of collected cells – affinity-based, size-based selection.
This circulating tumor DNA (ctDNA) market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the circulating tumor DNA (ctDNA) market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Circulating Tumor DNA (ctDNA) Market Scope
The circulating tumor DNA (ctDNA) market is segmented on the basis of method, sample, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Application
- Routine Screening
- Patient Work Up
- Early-Stage Disease
- Late Stage/Metastatic Disease
Sample
- Blood
- Urine
- Others
Method
- Sample Preparation
- Ultra-Low Passage Whole Genome Sequencing
- ctDNA Quantification
- Copy Number Analysis
- Statistical Analysis
End-Users
- Hospitals
- Research Laboratories
- Academia
- Research Centers
Circulating Tumor DNA (ctDNA) Market Regional Analysis
The circulating tumor DNA (ctDNA) market is analyzed and market size insights and trends are provided by country, method, sample, application and end-user as referenced above.
The countries covered in the circulating tumor DNA (ctDNA) market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the circulating tumor DNA (ctDNA) market, Because of the high frequency of cancer and strong demand for customised therapy.
Asia-Pacific is predicted to develop significantly during the forecast period of 2025 to 2032 due to a rise in government measures to enhance awareness.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Circulating Tumor DNA (ctDNA) Market Share
The circulating tumor DNA (ctDNA) market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to circulating tumor DNA (ctDNA) market.
Circulating Tumor DNA (ctDNA) Market Leaders Operating in the Market Are:
- GRAIL, Inc. (U.S)
- Guardant Health (U.S)
- Biodesix (U.S)
- Freenome Holdings, Inc. (U.S)
- LungLife AI, Inc. (U.S)
- Inivata Ltd (U.K)
- Personal Genome Diagnostics Inc. (U.S)
- CellMax Life (U.S)
- Vermillion Incorporated (U.S)
- Genomic Health Inc. (U.S)
- Foundation Medicine, Inc. (U.S)
- Biocept, Inc. (U.S)
- Myriad Genetics, Inc. (U.S)
- OncoCyte (U.S)
- Veracyte, Inc. (U.S)
Latest Developments in Circulating Tumor DNA (ctDNA) Market
- Biocept, Inc. announced the debut of its research-use-only (RUO) kits in January 2020, which are specifically developed for molecular laboratories to manage Biocept's Target Selector circulating tumor DNA (ctDNA) tests for liquid biopsy testing. This novel platform uses reagents, primers, and other technologies to enhance the number of specimens available for mutations of interest, resulting in higher assay sensitivity
- Roche stated in April 2021 that they would buy CAPP Medical to develop novel cancer technologies based on the detection of circulating tumor DNA (ctDNA) in blood. This acquisition will aid Roche in strengthening its market position and providing superior technology to its patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.