Global Classic Congenital Adrenal Hyperplasia Market
Market Size in USD Million
CAGR :
% 
 USD
284.31 Million
USD
563.19 Million
2024
2032
USD
284.31 Million
USD
563.19 Million
2024
2032
| 2025 –2032 | |
| USD 284.31 Million | |
| USD 563.19 Million | |
|
|
|
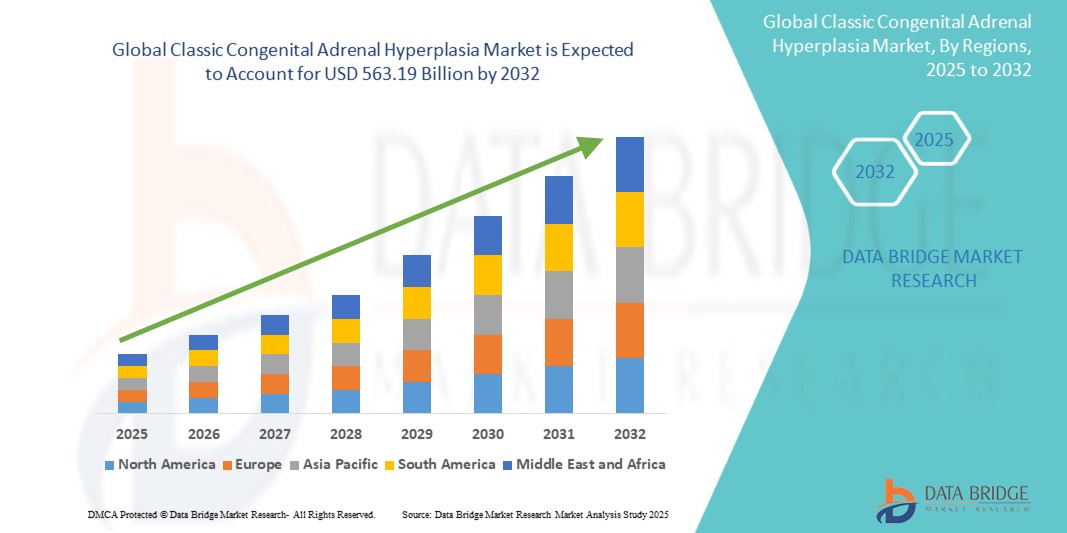
Classic Congenital Adrenal Hyperplasia Market Size
- The global Classic Congenital Adrenal Hyperplasia market was valued at USD 284.31 million in 2024 and is expected to reach USD 563.19 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 8.92%, primarily driven by the increasing diagnosis and treatment advancements
- This growth is driven by factors such as the Increasing awareness and early diagnosis and advancements in treatment options
Classic Congenital Adrenal Hyperplasia Market Analysis
- Classic Congenital Adrenal Hyperplasia (CAH) refers to a group of inherited disorders affecting the adrenal glands, leading to hormone imbalances. It is often caused by a deficiency in the enzyme 21-hydroxylase, which disrupts cortisol and aldosterone production, affecting the body's ability to regulate stress, salt balance, and reproductive functions
- The CAH market is experiencing significant growth, driven by an increased diagnosis rate and advancements in treatment options such as corticosteroid therapy and enzyme replacement
- Rising Awareness and Early Diagnosis: Growing awareness of CAH among healthcare providers and patients is leading to earlier diagnoses and better management of the disease, contributing to market growth
- Therapeutic Innovations such as innovative treatments, including gene therapy and synthetic corticosteroids, are improving patient outcomes, making them a significant factor in market expansion
- For Instance, A study titled “Effect of Newborn Screening for Congenital Adrenal Hyperplasia on the Diagnosis and Treatment of Newborns in Texas” in the U.S. revealed that early diagnosis and treatment of CAH in newborns drastically reduce the risk of severe complications, showing the importance of timely intervention and the role of the healthcare system in the market growth
Report Scope and Classic Congenital Adrenal Hyperplasia Market Segmentation
|
Attributes |
Classic Congenital Adrenal Hyperplasia Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Classic Congenital Adrenal Hyperplasia Market Trends
“Growing Adoption of Personalized and Precision Medicine”
- One notable trend in the Classic Congenital Adrenal Hyperplasia (CAH) market is the growing adoption of personalized and precision medicine. With advancements in genetic testing and a better understanding of the genetic mutations responsible for CAH, treatments are increasingly tailored to individual patients
- Personalized medicine allows for more effective management by optimizing hormone replacement therapies and dosages based on the patient's genetic profile and specific needs. This trend is further supported by innovations in biopharmaceuticals and targeted therapies, which aim to minimize side effects and improve long-term outcomes
- In addition, the development of new steroid alternatives and gene therapy approaches is gaining traction, offering hope for more permanent solutions to this condition. This shift towards personalized care is transforming how CAH is managed, leading to improved quality of life for patients
Classic Congenital Adrenal Hyperplasia Market Dynamics
Driver
“Increased Focus on Early Diagnosis and Newborn Screening Programs”
- A key driver of the Classic Congenital Adrenal Hyperplasia (CAH) market is the increased focus on early diagnosis and newborn screening programs. Early detection of CAH through routine newborn screening allows for timely treatment, which is crucial for preventing severe complications such as adrenal crises, growth issues, and fertility problems
- As more countries implement these screening programs, the number of diagnosed cases rises, leading to a greater demand for treatments such as corticosteroids and enzyme replacement therapies
- In addition, the growing awareness among healthcare providers about the importance of early diagnosis has led to more accurate and quicker identification of the condition, enabling prompt intervention. This rise in early diagnoses directly contributes to market growth by increasing both the number of patients seeking treatment and the need for advanced therapeutic options
For instance,
- In the U.S., the implementation of universal newborn screening for CAH has significantly reduced the incidence of adrenal crises in infants
- A study in Europe titled “Association of newborn screening of congenital adrenal hyperplasia with outcomes in the first 90 days of life: a multi-centre I-CAH registry analysis of contemporary practice” showed that early diagnosis of CAH in newborns allowed for the initiation of steroid therapy, resulting in better physical and psychological outcomes for patients
- The increasing focus on early diagnosis and awareness is significantly driving the CAH market by improving treatment outcomes, expanding diagnostic services, and increasing demand for therapeutic interventions, ultimately fueling market growth
Opportunity
“Development and Adoption of Gene Therapies”
- An emerging opportunity in the Classic Congenital Adrenal Hyperplasia (CAH) market lies in the development and adoption of gene therapies. Gene therapy aims to address the root cause of CAH by correcting the genetic mutations responsible for the enzyme deficiencies. This offers the potential for long-term or even permanent solutions, reducing the reliance on lifelong hormone replacement therapies. As research advances, clinical trials for gene therapies specific to CAH are gaining momentum, with promising results in pre-clinical and early-stage studies
- This opportunity is further supported by advances in CRISPR and gene-editing technologies, which offer precise methods to target and repair genetic mutations. The potential to offer curative treatments would not only revolutionize the management of CAH but could also significantly reduce treatment costs over time. As such, gene therapies present a compelling market opportunity for both biopharmaceutical companies and healthcare providers, contributing to substantial market growth in the coming years
For instance,
- In June 2021, researchers at the University of California, San Francisco, reported promising results from a study using CRISPR gene-editing technology to correct the genetic mutation causing CAH in animal models. The study demonstrated potential for a long-term solution to the enzyme deficiencies responsible for CAH, marking a significant milestone in gene therapy for the disorder
- In October 2022, a biopharmaceutical company, CRISPR Therapeutics, announced the initiation of Phase 1 clinical trials for a gene therapy approach aimed at treating CAH. This trial focuses on using gene-editing techniques to correct the genetic mutations responsible for the disease, offering the first clinical step towards a potential curative treatment for CAH patients
- The development of gene therapies for CAH holds the potential to revolutionize treatment by offering curative solutions. This innovation could significantly reduce long-term treatment costs, expand market demand for advanced therapies, and drive substantial growth in the CAH market
Restraint/Challenge
“High Cost of Treatment and Limited Access to Advanced Therapies”
- One major restraint in the Classic Congenital Adrenal Hyperplasia (CAH) market is the high cost of treatment and limited access to advanced therapies. While corticosteroids and enzyme replacement therapies are widely used to manage CAH, these treatments often require lifelong administration and can be costly
- In addition, emerging therapies, such as gene therapy, though promising, are still in early stages and are associated with high development costs, which could make them unaffordable for many patients. This financial burden can limit patient access to optimal care, particularly in low- and middle-income countries, further widening healthcare disparities
For instance,
- In April 2022, A report from the American Society of Pediatric Endocrinology highlighted that the cost of corticosteroid therapy for CAH patients could exceed USD 10,000 annually, creating a significant financial strain on families, particularly those without insurance coverage
- In January 2023, A clinical trial for gene therapy aimed at treating CAH by a biotech firm in Europe revealed that the cost per patient for the gene therapy treatment could be as high as USD 500,000, limiting widespread accessibility
- The high costs associated with treatment and limited access to advanced therapies could impede market growth by restricting patient access to effective care and slowing the adoption of newer, more expensive treatment options
Classic Congenital Adrenal Hyperplasia Market Scope
The market is segmented on the basis of type, treatment, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Treatment |
|
|
By End User |
|
Classic Congenital Adrenal Hyperplasia Market Regional Analysis
“North America is the Dominant Region in the Classic Congenital Adrenal Hyperplasia Market”
- North America is the dominant region in the Classic Congenital Adrenal Hyperplasia (CAH) market due to its advanced healthcare infrastructure, high levels of awareness about CAH, and widespread newborn screening programs. These factors enable early detection and timely treatment, leading to better patient outcomes
- U.S. is the leading country in this region. The U.S. benefits from cutting-edge medical research, the presence of major pharmaceutical companies, and significant healthcare spending. This allows for rapid development and availability of innovative treatments, including gene therapies, making it the dominant player in the CAH market
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia Pacific is expected to witness the highest growth rate in the Classic Congenital Adrenal Hyperplasia (CAH) market. This is due to increasing healthcare awareness, improving diagnostic capabilities, and growing healthcare investments in countries such as India and China
- In addition, the rising prevalence of genetic disorders and increasing adoption of newborn screening programs contribute to market expansion in the region
- China is expected to have the highest CAGR in the CAH market. The country is experiencing rapid healthcare advancements, expanding access to advanced diagnostics, and greater investment in medical research, enabling early diagnosis and better treatment availability
Classic Congenital Adrenal Hyperplasia Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Adrenas (U.S.)
- BridgeBio Inc. (U.S.)
- Crinetics Pharmaceuticals, Inc. (U.S.)
- Ferring (Switzerland)
- H. Lundbeck A/S (Denmark)
- IP Group PLC (U.K.)
- Lilly (U.S.)
- Merck & Co., Inc. (U.S.)
- Neurocrine Biosciences, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Spruce Biosciences, Inc. (U.S.)
- Sanofi (France)
- Zydus Group (India)
Latest Developments in Global Classic Congenital Adrenal Hyperplasia Market
- In January 2025, Crinetics Pharmaceuticals, Inc. announced positive topline results from an open-label, Phase 2 study of atumelnant, an investigational, once-daily oral adrenocorticotropic hormone (ACTH) receptor antagonist. The study focused on evaluating atumelnant for the treatment of classic congenital adrenal hyperplasia (CAH) and ACTH-dependent Cushing’s syndrome
- In January 2025, Neurocrine Biosciences, Inc. announced the publication of a supplement in The Journal of Clinical Endocrinology & Metabolism (JCEM), sponsored by the company, focused on Classic Congenital Adrenal Hyperplasia (CAH). Titled "Challenges and Opportunities in the Management of Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency Throughout the Lifetime," the supplement features eight review articles that offer a thorough examination of the clinical, psychosocial, treatment-related, and daily challenges faced by individuals living with classic CAH
- In December 2024, the U.S. Food and Drug Administration approved Crenessity (crinecerfont) for use in combination with glucocorticoids (steroids) to manage androgen (testosterone-like hormone) levels in adults and pediatric patients aged 4 years and older with classic congenital adrenal hyperplasia (CAH)
- In December, 2024, Spruce Biosciences, Inc. announced the topline results from its CAHmelia-204 study of tildacerfont in adult CAH and the CAHptain-205 study of tildacerfont in both adult and pediatric patients with Congenital Adrenal Hyperplasia. The CAHmelia-204 study was a Phase 2b, randomized, double-blind, placebo-controlled trial that assessed the safety and efficacy of tildacerfont in reducing supraphysiologic glucocorticoid usage in 100 adults with classic CAH
- In September 2024, BridgeBio Pharma, Inc. announced the topline results from the Phase 1/2 open-label ADventure study, which is evaluating BBP-631, the company's experimental adeno-associated virus (AAV) 5 gene therapy, for the treatment of congenital adrenal hyperplasia (CAH)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.