Global Clinical Next Generation Sequencing Nsg Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
8.92 Billion
USD
61.32 Billion
2025
2033
USD
8.92 Billion
USD
61.32 Billion
2025
2033
| 2026 –2033 | |
| USD 8.92 Billion | |
| USD 61.32 Billion | |
|
|
|
|
Clinical Next-Generation Sequencing (NSG) Testing Market Size
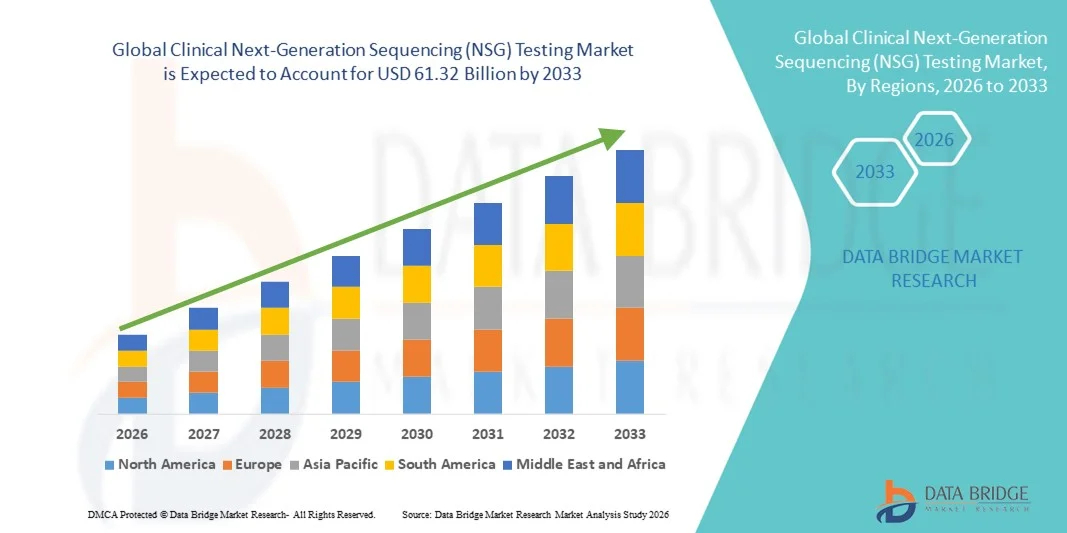
- The global clinical next-generation sequencing (NSG) testing market size was valued at USD 8.92 billion in 2025 and is expected to reach USD 61.32 billion by 2033, at a CAGR of 27.25% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced genomic technologies, rising demand for precision medicine, and growing awareness of genetic testing for early disease detection
- Furthermore, the expansion of clinical research, rising prevalence of genetic disorders and cancers, and the integration of NGS into routine diagnostics are accelerating the uptake of Clinical Next-Generation Sequencing (NGS) Testing solutions, thereby significantly boosting the industry's growth
Clinical Next-Generation Sequencing (NSG) Testing Market Analysis
- Clinical Next-Generation Sequencing (NGS) Testing, offering high-throughput genomic analysis for disease diagnosis, treatment selection, and research applications, is increasingly vital in both clinical and research settings due to its enhanced accuracy, speed, and scalability
- The escalating demand for NGS testing is primarily fueled by the rising prevalence of genetic disorders and cancers, growing adoption of precision medicine, and increasing integration of genomic technologies into routine diagnostics
- North America dominated the clinical next-generation sequencing (NGS) testing market with the largest revenue share of 40.1% in 2025, characterized by advanced healthcare infrastructure, high R&D spending, and a strong presence of key market players, with the U.S. experiencing substantial growth in NGS installations, particularly in hospitals, specialty clinics, and research institutions, driven by innovations from both established companies and startups focusing on faster, more cost-effective sequencing platforms
- Asia-Pacific is expected to be the fastest-growing region in the clinical next-generation sequencing (NGS) testing market during the forecast period due to increasing healthcare investment, rising awareness of genomic medicine, and the expansion of clinical and research facilities in countries such as China, India, and Japan
- The Sequencing segment dominated with the largest market revenue share of 45.3% in 2025, driven by the critical role of sequencing runs in all NGS workflows and the increasing number of clinical and research samples being processed globally
Report Scope and Clinical Next-Generation Sequencing (NSG) Testing Market Segmentation
|
Attributes |
Clinical Next-Generation Sequencing (NSG) Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Clinical Next-Generation Sequencing (NSG) Testing Market Trends
Enhanced Convenience Through Automation and Digital Integration
- A significant and accelerating trend in the global clinical next-generation sequencing (NGS) testing market is the increasing integration of advanced automation, robotics, and digital laboratory management solutions. These technologies are significantly enhancing operational efficiency, accuracy, and throughput in clinical and research laboratories
- For instance, several NGS platforms now incorporate automated sample preparation and sequencing workflows, reducing manual intervention and minimizing human error. Instruments from leading companies such as Illumina and Thermo Fisher Scientific offer integrated systems that streamline library preparation, sequencing, and data analysis, enabling laboratories to process a higher volume of samples with consistent quality
- Automation in NGS testing also enables real-time monitoring, remote access, and enhanced traceability of samples, supporting compliance with regulatory standards and improving reproducibility of results. Digital platforms facilitate laboratory-wide integration, allowing users to manage sample inventory, workflow scheduling, and sequencing operations through a single interface
- The seamless integration of automation with bioinformatics pipelines enhances data interpretation speed and accuracy, enabling clinicians and researchers to obtain actionable insights faster. These integrated systems support precision medicine initiatives, where rapid turnaround times and reliable sequencing results are critical for patient care
- This trend toward intelligent, automated, and interconnected NGS testing systems is reshaping laboratory workflows, reducing manual labor, and improving overall laboratory efficiency. Companies such as Agilent Technologies and QIAGEN are continually advancing automation features in their NGS platforms to meet the growing demand for high-throughput, reproducible, and clinically actionable results
- The demand for integrated and automated NGS solutions is growing rapidly across both clinical and research sectors, as laboratories increasingly prioritize efficiency, scalability, and data accuracy
Clinical Next-Generation Sequencing (NSG) Testing Market Dynamics
Driver
Rising Demand Due to Precision Medicine and Genomic Research Expansion
- The growing focus on precision medicine, oncology research, and genetic disorder screening is a major driver for the global Clinical NGS Testing market. Laboratories and hospitals are increasingly relying on NGS to provide comprehensive genomic insights for diagnosis, prognosis, and treatment selection
- For instance, in March 2024, Illumina launched its NovaSeq 2, an enhanced sequencing platform with faster processing times and higher throughput, enabling clinical laboratories to accelerate genomic testing and expand their testing portfolios. Such innovations are expected to drive market growth in the forecast period
- As healthcare providers and research institutions demand faster, more accurate, and scalable sequencing solutions, NGS platforms that integrate automated workflows and advanced bioinformatics are becoming indispensable
- Furthermore, increasing investments in genomic research, expansion of molecular diagnostics programs, and the rising prevalence of genetic diseases are contributing to the growing adoption of Clinical NGS Testing systems
- The ability to perform multi-gene panels, whole-exome, and whole-genome sequencing with high precision supports personalized treatment approaches, which is a critical factor driving market demand
- Laboratories are also adopting NGS solutions to improve operational efficiency, reduce turnaround time, and enhance overall sample throughput, further strengthening the market trajectory
Restraint/Challenge
High Capital Costs and Technical Expertise Requirements
- The relatively high cost of NGS platforms, consumables, and associated software can be a barrier to adoption, particularly for smaller laboratories or institutions in developing regions
- For instance, advanced sequencing platforms from companies such as Thermo Fisher Scientific and Illumina can involve substantial upfront investment, including hardware, software, and automation integration, which may deter price-sensitive laboratories from adopting the technology
- In addition, operating NGS systems requires specialized technical expertise for sample preparation, sequencing, and data analysis, posing challenges for laboratories with limited skilled personnel. Training and hiring qualified staff can increase operational costs
- Ensuring data accuracy and compliance with regulatory standards also demands continuous quality control and validation processes, which can be resource-intensive
- Overcoming these challenges through scalable solutions, affordable entry-level platforms, and enhanced training programs for laboratory personnel is essential for broader adoption. Companies are addressing these barriers by developing modular systems, cloud-based analysis platforms, and streamlined automation to lower costs and simplify operations
- While NGS testing adoption is steadily increasing, managing operational complexity, balancing costs, and ensuring consistent data quality remain critical challenges that laboratories and solution providers must address for sustained market growth
Clinical Next-Generation Sequencing (NSG) Testing Market Scope
The market is segmented on the basis of technology, application, end user, service, and workflow.
- By Technology
On the basis of technology, the Clinical Next-Generation Sequencing (NSG) Testing market is segmented into Sequencing By Synthesis, Ion Semiconductor Sequencing, Single-Molecule Real-time Sequencing, Nano Pore Sequencing, and Other Sequencing Technologies. The Sequencing By Synthesis segment dominated the largest market revenue share of 42.6% in 2025, driven by its high accuracy, scalability, and ability to generate large volumes of genomic data efficiently. It is extensively used in clinical diagnostics, oncology research, rare disease profiling, and large-scale genomic studies. Hospitals and academic research centers prefer this technology for its compatibility with multiple sample types, reliable bioinformatics pipelines, and ability to provide actionable insights across human, microbial, and plant genomes.
The Ion Semiconductor Sequencing segment is anticipated to witness the fastest CAGR of 22.4% from 2026 to 2033, fueled by rapid sequencing turnaround, lower operational costs, and growing adoption in drug discovery and agricultural research. Its real-time data generation and easy integration into lab workflows make it increasingly popular among biotech firms and clinical laboratories for precision medicine and targeted therapeutics research. Moreover, its compact instrument design and scalability for medium-throughput projects make it suitable for a wide range of laboratory settings. Increasing investments in genomics and personalized medicine initiatives are also driving the accelerated adoption of this technology globally.
- By Application
On the basis of application, the market is segmented into Diagnostics, Drug Discovery, Agricultural and Animal Research, and Other Applications. The Diagnostics segment accounted for the largest market revenue share of 44.8% in 2025, owing to the growing utilization of NGS in early disease detection, cancer profiling, and personalized treatment planning. Clinical laboratories and hospitals leverage NGS-based diagnostics to provide actionable insights and ensure better patient outcomes. The segment’s dominance is supported by increasing prevalence of genetic disorders, rising cancer incidence, and the need for precision medicine solutions. Integration of NGS into routine clinical workflows is becoming standard practice in many developed countries. The availability of advanced NGS platforms with faster turnaround and high accuracy further fuels adoption. Healthcare providers are increasingly investing in genomics infrastructure to deliver timely and reliable diagnostic services. Collaborations between hospitals, research institutes, and diagnostic companies are expanding the reach of NGS applications. Regulatory approvals and reimbursement support in key regions are also encouraging adoption. With continuous technological advancements, NGS diagnostics are becoming more cost-effective, scalable, and accessible, strengthening their market leadership. Rising awareness among clinicians and patients regarding personalized healthcare and preventive strategies continues to drive demand.
The Drug Discovery segment is expected to witness the fastest CAGR of 21.5% from 2026 to 2033, driven by the increasing need for genomic insights in biomarker discovery, target validation, and development of precision therapeutics. Pharmaceutical companies are adopting NGS in preclinical and clinical workflows to streamline drug development and enhance efficiency. The fast growth is further supported by the expansion of precision medicine initiatives and the demand for more targeted and effective therapies. Integration of NGS in high-throughput screening and compound profiling enables faster identification of novel drug targets. Collaborations between biotech firms and research organizations are accelerating adoption. Advancements in sequencing technology, automation, and bioinformatics analysis reduce operational costs and increase data reliability. Rising R&D expenditure by pharmaceutical companies globally is bolstering the implementation of NGS in drug discovery pipelines. Increasing focus on rare diseases, oncology therapeutics, and immunotherapy research further propels market growth. The segment’s adoption is also supported by government funding and strategic partnerships to accelerate innovation. NGS is being integrated into multi-omics approaches, providing comprehensive insights into molecular mechanisms. Overall, the segment is expected to maintain strong momentum due to its critical role in enhancing drug discovery efficiency and enabling personalized therapy development.
- By End User
On the basis of end user, the market is segmented into Academic Institutes and Research Centres, Hospitals and Clinics, Pharmaceutical and Biotechnology Companies, and Other End Users. Academic Institutes and Research Centres dominated the market with a revenue share of 40.7% in 2025, supported by government-funded genomics programs, ongoing research initiatives, and collaborations with biotechnology companies. These institutions are leading efforts in population genomics, functional genomics, and translational research. The segment benefits from access to advanced sequencing infrastructure, skilled personnel, and extensive research networks. Academic research also drives innovation in sequencing workflows, data analysis pipelines, and clinical applications. Long-term funding and strategic partnerships with pharma and diagnostics companies strengthen the adoption of NGS platforms. Increasing focus on multi-omics research, precision medicine projects, and public health initiatives underpins market dominance. Expansion of genomics centers and dedicated NGS facilities across universities is fueling the segment. Rising publication outputs, research grants, and participation in global consortia continue to reinforce growth. Knowledge sharing and technology transfer from academic institutes to industry partners also contribute to widespread adoption.
Pharmaceutical and Biotechnology Companies are expected to register the fastest CAGR of 23.1% from 2026 to 2033, propelled by their growing reliance on NGS for drug discovery, biomarker validation, and clinical trial support. Rapid advancements in NGS technology and analytics facilitate accelerated drug development and precision therapeutics research. Biopharmaceutical firms are increasingly integrating NGS into target discovery, companion diagnostics, and pharmacogenomic studies. Strategic partnerships with sequencing technology providers enable streamlined workflows and cost optimization. Adoption is also driven by the need to comply with regulatory requirements for genomic-based therapies. Increased focus on oncology, rare diseases, and immunotherapies fuels demand. Expansion into emerging markets and global clinical trial operations further supports growth. The companies leverage NGS to accelerate decision-making, optimize candidate selection, and reduce time-to-market for new drugs. Automation, cloud-based data management, and AI-driven analytics enhance efficiency and data accuracy. Investment in proprietary NGS platforms and collaborations with CROs strengthens competitive advantage. Rising demand for personalized medicine and targeted treatments continues to sustain the high growth trajectory of this segment.
- By Service
On the basis of service, the market is segmented into Human Genome Sequencing, Single Cell Sequencing, Microbial Genome-based Sequencing, Gene Regulation Services, Animal and Plant Sequencing, and Other Sequencing Services. Human Genome Sequencing dominated the market with a revenue share of 41.9% in 2025, driven by the increasing focus on precision medicine, population genomics studies, and disease profiling applications. The demand is particularly high in clinical and research institutions aiming to generate actionable insights from genome data. Hospitals, diagnostic laboratories, and research centers are leveraging Human Genome Sequencing for identifying genetic predispositions, optimizing treatment plans, and supporting drug development. The growing availability of cost-effective sequencing platforms has further accelerated adoption. Government initiatives and funding programs for large-scale genomic studies support the segment’s growth. Collaborations between academic institutes and biotech firms are also expanding the reach of human genome projects. Increasing awareness of genetic testing and preventive healthcare fuels the segment’s dominance. Advancements in high-throughput sequencing technology and improved data accuracy are attracting new end users. In addition, the integration of cloud-based storage and AI-driven analysis platforms is enhancing workflow efficiency. Human Genome Sequencing continues to remain central to personalized medicine, clinical diagnostics, and translational research worldwide.
Single Cell Sequencing is expected to witness the fastest CAGR of 24.2% from 2026 to 2033, due to its rising adoption in cancer research, immunology studies, and investigations of cellular heterogeneity. Its ability to provide highly detailed cellular-level information is making it indispensable for advanced genomic research. Pharmaceutical and biotechnology companies are increasingly employing single cell approaches to understand disease mechanisms and identify novel drug targets. The segment’s growth is supported by the development of specialized instruments, reagents, and bioinformatics software tailored for single cell applications. Academic research institutions are rapidly adopting single cell techniques for multi-omics studies, including transcriptomics and epigenomics. The technology’s capability to resolve cellular diversity in complex tissues drives demand across oncology, neurology, and immunology studies. Increasing collaborations between sequencing service providers and research labs are expanding adoption. Rising awareness of the importance of cellular heterogeneity in disease progression further propels market growth. Technological advancements, such as droplet-based and microfluidic platforms, improve throughput and reduce costs. The segment’s fast growth trajectory is further fueled by integration with AI-driven analytical platforms for enhanced data interpretation.
- By Workflow
On the basis of workflow, the market is segmented into Pre-Sequencing, Sequencing, and Data Analysis. The Sequencing segment dominated with the largest market revenue share of 45.3% in 2025, driven by the critical role of sequencing runs in all NGS workflows and the increasing number of clinical and research samples being processed globally. Laboratories across hospitals, research institutes, and biopharmaceutical companies are expanding sequencing capacity to meet rising sample volumes. The segment benefits from continuous technological innovations, including higher-throughput sequencers, improved chemistry, and faster runtime. Sequencing forms the backbone of NGS studies, enabling genomics-based diagnostics, drug discovery, and population studies. Increasing adoption in precision medicine and large-scale population genomics projects supports sustained demand. Integration with automated sample preparation and library construction systems enhances efficiency. Government and private research funding for genomics initiatives also underpin growth. The segment’s dominance is strengthened by the availability of comprehensive sequencing platforms and associated reagents. In addition, user-friendly interfaces and robust technical support from instrument providers encourage widespread adoption.
The Data Analysis segment is expected to witness the fastest CAGR of 22.8% from 2026 to 2033, fueled by the growing need for advanced bioinformatics, AI-enabled variant calling, and efficient interpretation of the massive volumes of sequencing data generated. Increasing complexity of NGS datasets necessitates sophisticated analytical pipelines. Clinical laboratories and research organizations are adopting AI-driven and cloud-based bioinformatics platforms to accelerate insights. Demand for multi-omics integration, functional annotation, and predictive modeling further drives growth. The segment benefits from the rise in personalized medicine, where data-driven treatment decisions are essential. Investment in bioinformatics infrastructure, software, and skilled personnel is expanding globally. Collaborations between sequencing service providers and software developers improve analytical efficiency. The growing adoption of automated workflows and standardized data pipelines reduces turnaround time and enhances reproducibility. Data security, scalability, and interoperability are also critical factors boosting segment growth. Overall, data analysis is emerging as a vital component of the NGS workflow, ensuring actionable insights from high-volume genomic data.
Clinical Next-Generation Sequencing (NSG) Testing Market Regional Analysis
- North America dominated the clinical next-generation sequencing (NGS) testing market with the largest revenue share of 40.1% in 2025
- Characterized by advanced healthcare infrastructure, high R&D spending, and a strong presence of key market players. The region is witnessing substantial growth in NGS installations across hospitals, specialty clinics, and research institutions, driven by innovations from both established companies and startups focusing on faster, more cost-effective sequencing platforms
- The widespread adoption is further supported by a technologically advanced healthcare ecosystem, skilled workforce, and the growing demand for precision medicine and genomic research, establishing North America as the leading region in the Clinical NGS Testing market
U.S. Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The U.S. clinical next-generation sequencing (NGS) testing market captured the largest revenue share within North America in 2025, fueled by the well-established healthcare and research ecosystem, strong pharmaceutical and biotechnology sectors, and rapid adoption of laboratory automation and high-throughput sequencing solutions. Hospitals, specialty clinics, and research institutions are increasingly implementing NGS platforms to accelerate genomic diagnostics, support oncology and rare disease testing, and enhance clinical research capabilities. The combination of robust investments in genomic medicine and a growing pipeline of innovative sequencing technologies continues to drive market growth in the U.S.
Europe Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The Europe clinical next-generation sequencing (NGS) testing market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising adoption of genomic medicine, supportive healthcare policies, and increasing investments in clinical and academic research. Countries such as the U.K. and Germany are witnessing growing integration of NGS platforms across hospitals, research centers, and diagnostic laboratories, improving patient care and research efficiency.
U.K. Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The U.K. clinical next-generation sequencing (NGS) testing market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by expanding genomic research initiatives, rising precision medicine adoption, and supportive healthcare infrastructure. Hospitals and research institutions are increasingly deploying NGS platforms to facilitate rapid and accurate genomic testing.
Germany Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The Germany clinical next-generation sequencing (NGS) testing market is expected to expand at a considerable CAGR during the forecast period, fueled by strong healthcare infrastructure, increasing adoption of genomics in clinical practice, and rising investments in research and development. The availability of automated and high-throughput sequencing platforms supports the growing demand from hospitals, specialty clinics, and research laboratories.
Asia-Pacific Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The Asia-Pacific clinical next-generation sequencing (NGS) testing market is poised to grow at the fastest CAGR during the forecast period, driven by increasing healthcare investments, rising awareness of genomic medicine, and rapid expansion of clinical and research facilities in countries such as China, India, and Japan. Government initiatives to improve access to genomic technologies, along with growth in hospital and research infrastructure, are supporting strong market adoption across the region.
Japan Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The Japan clinical next-generation sequencing (NGS) testing market is gaining momentum due to advanced healthcare technologies, increasing focus on precision medicine, and expanding research infrastructure. Hospitals and clinical laboratories are adopting high-throughput sequencing platforms to support oncology diagnostics, rare disease testing, and pharmacogenomics applications.
China Clinical Next-Generation Sequencing (NGS) Testing Market Insight
The China clinical next-generation sequencing (NGS) testing market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid expansion of healthcare and research infrastructure, increasing investments in genomic medicine, and growing adoption of high-throughput NGS platforms across hospitals, diagnostic labs, and research facilities. Government support for genomic research and strong domestic sequencing solution providers are key factors propelling the market in China.
Clinical Next-Generation Sequencing (NSG) Testing Market Share
The Clinical Next-Generation Sequencing (NSG) Testing industry is primarily led by well-established companies, including:
• Illumina (U.S.)
• Thermo Fisher Scientific (U.S.)
• Pacific Biosciences (U.S.)
• BGI Genomics (China)
• Roche (Switzerland)
• Agilent Technologies (U.S.)
• Oxford Nanopore Technologies (U.K.)
• PerkinElmer (U.S.)
• GenapSys (U.S.)
• 10x Genomics (U.S.)
• MGI Tech (China)
• Novogene (China)
• SeqWell (U.S.)
• Fulgent Genetics (U.S.)
• Macrogen (South Korea)
Latest Developments in Global Clinical Next-Generation Sequencing (NSG) Testing Market
- In July 2023, QIAGEN expanded its next‑generation sequencing portfolio with the launch of its QIAseq Normalizer Kit, which speeds up DNA library normalization to ~30 minutes from several hours, and is automation‑friendly with high‑throughput (96‑sample) capability
- In October 2024, Illumina announced a strategic partnership with Broad Institute to develop new gene sequencing kits leveraging CRISPR‑based PerturbSeq technology, aimed at enabling large‑scale single‑cell and gene‑network screening for disease research
- In May 2025, Roche unveiled its proprietary “Sequencing by Expansion (SBX)” technology, a new class of NGS platform designed for higher throughput, longer reads, and greater flexibility—marking a significant step forward in clinical NGS instrument performance
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.