Global Clinical Reference Laboratory Market
Market Size in USD Billion
CAGR :
% 
 USD
2.71 Billion
USD
4.93 Billion
2024
2032
USD
2.71 Billion
USD
4.93 Billion
2024
2032
| 2025 –2032 | |
| USD 2.71 Billion | |
| USD 4.93 Billion | |
|
|
|
|
Global Clinical Reference Laboratory Market Analysis
The global clinical reference laboratory market is experiencing steady growth, driven by the increasing demand for accurate diagnostic tests, advancements in genetic testing, and the rise of personalized medicine. Clinical reference laboratories provide high-quality diagnostic testing services, such as blood and urine tests, genetic testing, and disease-specific analyses, which are essential in disease management and prevention. Growing chronic disease prevalence and aging populations globally are key factors contributing to the market's expansion. The market is also benefiting from innovations in molecular diagnostics and the increasing adoption of point-of-care testing. Technological advancements, such as automation and artificial intelligence integration, are further improving test accuracy and turnaround times, which enhances laboratory services.
Global Clinical Reference Laboratory Market Size
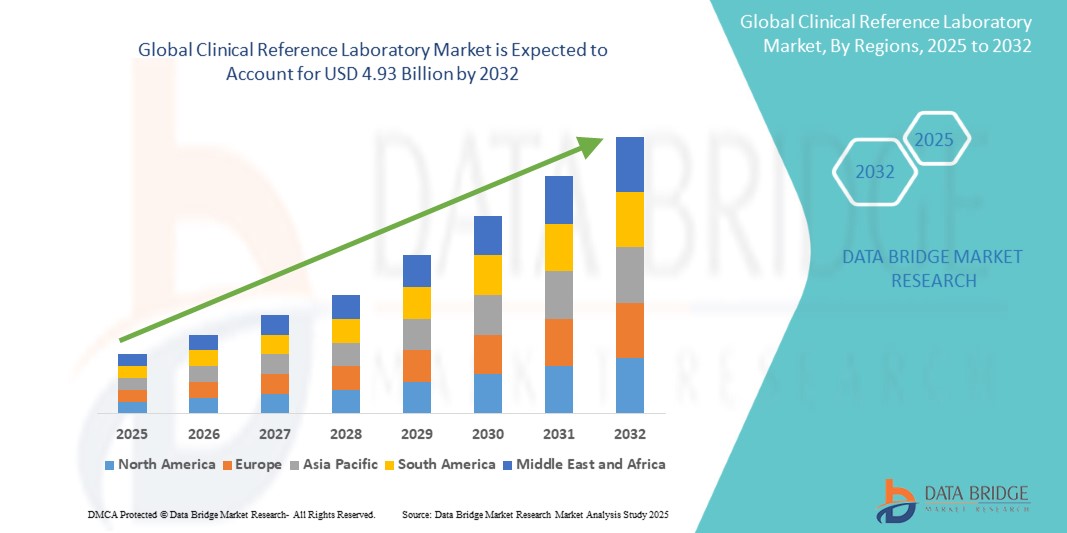
Global clinical reference laboratory market size was valued at USD 2.71 billion in 2024 and is projected to reach USD 4.93 billion by 2032, with a CAGR of 9.1% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Global Clinical Reference Laboratory Market Trends
“Integration of AI and Automation in Laboratory Services”
A prominent trend in the global clinical reference laboratory market is the integration of artificial intelligence (AI) and automation technologies. Laboratories are increasingly adopting automated systems for sample processing, testing, and data analysis, which enhances operational efficiency and accuracy. AI applications, such as machine learning models for interpreting complex test results, are improving the speed and precision of diagnostics. This trend is particularly important for genetic testing, where high-throughput analysis of genomic data can significantly benefit from AI capabilities. As AI and automation technologies evolve, they are expected to drive further innovation in clinical laboratory services, allowing for more personalized and cost-effective testing solutions.
Global Digital Genome Market Segmentation
|
Attributes |
Global Clinical Reference Laboratory Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Quest Diagnostics (U.S.), LabCorp (U.S.), Eurofins Scientific (Luxembourg), Medicover (Sweden), Sonic Healthcare (Australia), Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Abbott Laboratories (U.S.), and BioReference Laboratories, Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Global Clinical Reference Laboratory Market Definition
A clinical reference laboratory is a specialized medical laboratory that performs high-volume diagnostic testing, typically on specimens referred by hospitals, clinics, or other healthcare providers. These laboratories offer advanced, specialized testing services that may not be available in standard hospital laboratories. They play a critical role in diagnosing diseases, monitoring health conditions, and supporting medical research.
Global Clinical Reference Laboratory Market
Drivers
- Increased Demand for Accurate Diagnostic Testing
The growing need for accurate and reliable diagnostic testing is one of the primary drivers of the global clinical reference laboratory market. With the rising prevalence of chronic conditions such as diabetes, cardiovascular diseases, and neurological disorders, as well as the increasing incidence of infectious diseases and cancers, there is an increasing demand for precise diagnostic tools. Timely and accurate diagnosis is critical for effective disease management, especially in the case of complex and life-threatening conditions. Clinical reference laboratories provide essential diagnostic services that contribute to early detection and intervention, allowing for better patient outcomes. Furthermore, as healthcare systems worldwide emphasize preventive care, the need for diagnostic tests that identify health conditions at an early stage is growing. This trend is especially pronounced in regions with rapidly aging populations, where early detection and intervention are key to managing healthcare costs and improving quality of life. The demand for diagnostic tests is also bolstered by the growing awareness among the general population regarding the importance of regular health check-ups. As individuals become more proactive about their health, there is an increased reliance on diagnostic tests to detect diseases in their early stages, further contributing to the market's expansion. In addition, the COVID-19 pandemic underscored the importance of diagnostic testing, especially for infectious diseases, driving significant investment in testing infrastructure and the adoption of diagnostic services, which continues to have a lasting impact on the clinical reference laboratory market.
- Technological Advancements in Molecular and Genetic Testing
Technological advancements in molecular diagnostics and genetic testing are transforming clinical reference laboratories and driving the market's growth. Techniques such as high-throughput sequencing, polymerase chain reaction (PCR)-based diagnostics, and next-generation sequencing (NGS) have revolutionized the ability to detect and understand diseases at a molecular level. These innovations allow laboratories to perform more detailed and accurate tests, enabling the detection of genetic mutations, rare diseases, and pathogens that were previously difficult to identify. In particular, the demand for genetic testing is on the rise, fueled by the increasing prevalence of inherited genetic disorders, as well as the expanding applications of genetic testing in oncology, prenatal care, and pharmacogenomics. Molecular diagnostics also offer the ability to detect infections more quickly and accurately, including emerging infectious diseases, thus playing a crucial role in managing public health threats. With the growing demand for personalized medicine, which relies on genetic and molecular data to tailor treatments to individual patients, clinical reference laboratories are at the forefront of providing the necessary testing services that enable healthcare providers to offer customized treatment plans. As these technologies continue to improve, they will not only enhance diagnostic accuracy but also make advanced testing more accessible and cost-effective, further driving the demand for clinical reference laboratory services.
Opportunities
- Expansion of Healthcare Infrastructure in Emerging Markets
The rapid expansion of healthcare infrastructure in emerging markets presents significant opportunities for the global clinical reference laboratory market. As countries in Asia-Pacific, Latin America, and parts of Africa invest heavily in healthcare infrastructure, access to high-quality diagnostic services is improving, creating a strong demand for clinical reference laboratory services. The growing middle class in these regions, along with increased government spending on healthcare, is contributing to better access to healthcare and diagnostic services. In addition, these regions are experiencing a rising burden of both infectious diseases and non-communicable diseases, such as cancer and diabetes, which are driving the need for advanced diagnostic services. The improvement in diagnostic capabilities in these regions, coupled with the expansion of laboratory networks, will contribute to greater adoption of reference laboratory services. Clinical reference laboratories are crucial in addressing the need for high-quality, accurate, and timely diagnostic tests, which can significantly impact disease outcomes. This growth in healthcare access presents a major opportunity for laboratory service providers to expand their operations and capitalize on the increased demand for diagnostic services in emerging markets.
- Advances in Personalized and Precision Medicine
Another key opportunity in the clinical reference laboratory market lies in the rise of personalized and precision medicine. As healthcare continues to evolve toward more tailored treatment approaches, the need for diagnostic tests that provide detailed information about a patient’s genetic makeup, disease risk factors, and response to potential treatments has become more prominent. Personalized medicine, which aims to provide treatments based on an individual’s genetic profile, is increasingly becoming a standard approach in oncology, cardiology, and other therapeutic areas. Clinical reference laboratories are essential in this context, as they play a pivotal role in identifying genetic biomarkers and disease-specific characteristics that inform treatment decisions. Laboratories that offer specialized molecular diagnostics, including genetic testing, pharmacogenomic profiling, and tumor genomics, are well-positioned to support the growing demand for personalized therapies. As more healthcare providers embrace precision medicine, the demand for advanced diagnostic services will continue to rise, creating opportunities for clinical reference laboratories to expand their service offerings and meet the needs of both healthcare providers and patients.
Restraints/Challenges
- High Costs of Advanced Testing Services
One of the major challenges facing the global clinical reference laboratory market is the high cost of specialized testing services, particularly those related to genetic and molecular diagnostics. Advanced testing methods, such as next-generation sequencing, require expensive equipment, highly trained personnel, and compliance with strict regulatory standards, all of which contribute to the overall cost of testing. This cost can be prohibitive, especially in low- and middle-income countries where healthcare budgets are limited and access to advanced healthcare services is restricted.
Moreover, the financial burden of diagnostic testing can be a significant barrier for patients, particularly in regions where health insurance coverage for advanced testing is limited or unavailable. While the increasing availability of these tests is driving innovation, the costs associated with their delivery continue to be a challenge that limits widespread adoption. To address this issue, clinical reference laboratories will need to explore cost-reduction strategies, such as automation, streamlining operations, and adopting more affordable testing technologies, in order to make their services more accessible to a broader patient population.
- Stringent Regulatory Requirements
Clinical reference laboratories are subject to stringent regulatory standards that govern testing accuracy, data privacy, and laboratory operations. Compliance with these regulations is crucial for ensuring the reliability of test results and maintaining patient safety, but navigating these complex requirements can be time-consuming and costly. Laboratories must adhere to local and international regulations, such as those established by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Clinical Laboratory Improvement Amendments (CLIA).
In addition to meeting the regulatory standards for diagnostic accuracy, laboratories must also ensure the protection of patient data in compliance with data privacy laws such as the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in the European Union. The process of obtaining and maintaining certifications, conducting regular audits, and investing in technologies to ensure compliance adds to the operational costs and can delay the rollout of new testing services. These regulatory challenges present significant hurdles for clinical reference laboratories, particularly in regions with evolving or complex regulatory frameworks.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Clinical Reference Laboratory Market Scope
The market is segmented on the basis of service type and end-user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Service Type
- Testing Services
- Genetic Testing
- Blood Testing
- Urine Testing
End-User
- Hospitals,
- Diagnostic Centers
- Research Institutions
- Pharmaceutical Companies
Global Clinical Reference Laboratory Market Regional Analysis
The market is analysed and market size insights and trends are provided of country, service type, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the clinical reference laboratory market, driven by advanced healthcare infrastructure, high demand for diagnostic services, and technological innovation. The U.S. remains a key contributor, with widespread access to state-of-the-art diagnostic tools and testing services. The presence of major diagnostic companies, ongoing clinical research, and high healthcare spending are key factors contributing to the growth of the market in this region. The growth in healthcare investments and increased adoption of personalized medicine further solidify North America’s position as a leader in the clinical reference laboratory market.
The Asia-Pacific region is expected to experience the highest growth rate in the clinical reference laboratory market. This growth is fueled by expanding healthcare investments, rising disease burdens, and the increasing demand for diagnostic services in countries like China, India, and Japan. As these countries modernize their healthcare infrastructure and improve access to healthcare, the clinical reference laboratory market in Asia-Pacific is poised for rapid expansion. The Middle East and Africa are also witnessing growth as healthcare systems improve, although adoption rates remain relatively low in some regions due to financial and infrastructural constraints.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Global Clinical Reference Laboratory Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Global Clinical Reference Laboratory Market Leaders Operating in the Market Are:
- Quest Diagnostics (U.S.)
- LabCorp (U.S.)
- Eurofins Scientific (Luxembourg)
- Medicover (Sweden)
- Sonic Healthcare (Australia)
- Roche Diagnostics (Switzerland)
- Siemens Healthineers (Germany)
- Abbott Laboratories (U.S.)
- BioReference Laboratories, Inc. (U.S.)
Latest Developments in Global Clinical Reference Laboratory Market
- In August 2023, the American Institute of Pathology & Laboratory Sciences (Ampath) opened its first reference laboratory in Gurgaon, expanding its presence in North India and marking its second reference lab in the country. This facility enhanced Ampath's capacity to provide high-quality diagnostic services, improving access to timely pathology and laboratory services for healthcare providers and patients in the region
- In July 2020, Synlab and Microba formed a partnership to launch the gut microbiome test MyBiome in Europe and Latin America. This innovative metagenomic sequence-based test offers comprehensive, actionable insights into individuals’ gut microbiomes. The collaboration enhanced SYNLAB’s diagnostic portfolio, positioning it as a leader in microbiome testing and meeting the growing demand for personalized healthcare solutions in clinical reference laboratories
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.