Global Cosmetovigilance Market
Market Size in USD Billion
CAGR :
% 
 USD
12.26 Billion
USD
25.84 Billion
2025
2033
USD
12.26 Billion
USD
25.84 Billion
2025
2033
| 2026 –2033 | |
| USD 12.26 Billion | |
| USD 25.84 Billion | |
|
|
|
|
Cosmetovigilance Market Size
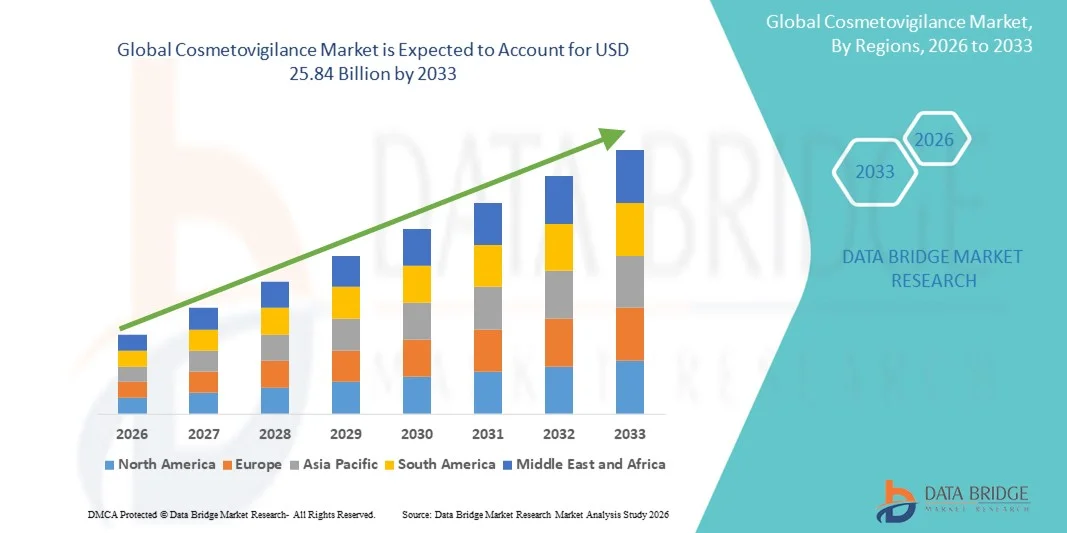
- The global Cosmetovigilance market size was valued at USD 12.26 billion in 2025 and is expected to reach USD 25.84 billion by 2033, at a CAGR of 9.77% during the forecast period
- The market growth is largely fueled by increasing regulatory scrutiny on cosmetic product safety, rising consumer awareness about adverse effects, and the growing demand for transparent safety monitoring across personal care and beauty products in both developed and emerging markets
- Furthermore, the rising emphasis on post-marketing surveillance, adverse event reporting, and risk assessment by manufacturers and regulatory authorities is establishing cosmetovigilance as a critical component of the cosmetic and personal care industry, thereby significantly boosting the market’s growth
Cosmetovigilance Market Analysis
- The Cosmetovigilance market, focused on monitoring, assessing, and preventing adverse effects related to cosmetic and personal care products, is becoming an increasingly critical part of the global beauty and wellness industry due to rising safety concerns, regulatory requirements, and heightened consumer awareness regarding product ingredients and side effects
- The escalating demand for cosmetovigilance is primarily fueled by stricter global cosmetic regulations, the increasing number of new product launches, growing cases of skin reactions and allergies, and the rising importance placed on post-marketing surveillance by both authorities and manufacturers
- North America dominated the cosmetovigilance market with the largest revenue share of 43% in 2025, characterized by advanced regulatory frameworks, strong presence of leading cosmetic manufacturers, high consumer awareness, and a well-established infrastructure for adverse event reporting, with the U.S. experiencing substantial growth in cosmetovigilance implementation across both mass-market and premium product segments
- Asia-Pacific is expected to be the fastest growing region in the cosmetovigilance market during the forecast period, registering a strong CAGR, due to rapid urbanization, expanding middle-class population, increasing consumption of beauty and personal care products, growing regulatory focus on product safety, and rising awareness of cosmetovigilance practices in countries such as China, India, Japan, and South Korea
- The post-marketing services segment dominated the largest market revenue share of 61.4% in 2025, driven by the growing regulatory requirement to continuously monitor, assess, and report adverse events related to cosmetic products after they are launched in the market
Report Scope and Cosmetovigilance Market Segmentation
|
Attributes |
Cosmetovigilance Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• IQVIA (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cosmetovigilance Market Trends
Rising Focus on Post-Market Surveillance and Regulatory Compliance
- A significant and accelerating trend in the global Cosmetovigilance market is the strengthening focus on post-market surveillance and compliance with increasingly strict cosmetic safety regulations across major regions such as Europe, North America, and parts of Asia. Regulatory authorities are reinforcing the need for continuous monitoring of cosmetic products even after they have entered the market, which is intensifying demand for structured cosmetovigilance systems
- For instance, under the EU Cosmetic Regulation (EC) No 1223/2009, companies are obligated to report serious undesirable effects (SUEs) linked to cosmetic products, encouraging manufacturers to invest in advanced surveillance frameworks to ensure proper documentation and traceability
- The rising incidence of allergic reactions, skin irritation, and long-term side effects from cosmetic and personal care products has further increased the need for efficient safety monitoring mechanisms. Companies are now prioritizing real-time adverse event reporting and stronger product safety data collection processes to protect both consumers and brand credibility
- In addition, the growing consumer awareness regarding ingredient safety, cruelty-free testing, and transparency in cosmetic formulations is encouraging brands to adopt comprehensive cosmetovigilance strategies that help them demonstrate safety, accountability, and product reliability
- The expansion of global cosmetic trade and the increasing number of product launches each year are also contributing to this trend, as a higher volume of products in circulation naturally increases the need for effective monitoring and risk-management solutions
- This industry shift towards proactive safety oversight is reshaping how cosmetic companies approach quality control, regulatory documentation, and long-term brand trust building, thereby significantly strengthening the role of cosmetovigilance in the overall product lifecycle
Cosmetovigilance Market Dynamics
Driver
Growing Need Due to Increasing Adverse Event Reporting and Consumer Safety Awareness
- The increasing number of reported adverse reactions associated with cosmetic and personal care products is a key driver fueling the growth of the Cosmetovigilance market globally
- For instance, in May 2024, health authorities in multiple regions reinforced guidelines for reporting cosmetic-related side effects, compelling manufacturers, importers, and distributors to adopt formal cosmetovigilance systems for tracking and analyzing adverse events
- As consumers become more attentive to the safety and quality of cosmetics they use daily, there is a rising demand for accountability, transparency, and scientific backing regarding product safety. This growing awareness is motivating companies to invest in advanced safety monitoring programs
- Furthermore, the rapid expansion of the cosmetics and skincare industry — especially in emerging markets — has significantly increased product usage, directly contributing to a higher volume of safety data that must be monitored, analyzed, and reported systematically
- The increasing popularity of online cosmetic sales and cross-border product distribution is also making adverse effect tracking more complex, creating a strong need for centralized and structured cosmetovigilance frameworks
- These combined factors, including regulatory mandates, rising case volumes, and heightened consumer consciousness, are significantly accelerating the demand for specialized cosmetovigilance solutions across the global market
Restraint/Challenge
Limited Awareness, Underreporting, and High Implementation Costs
- Despite the growing importance of cosmetovigilance, limited awareness among small and medium-sized cosmetic manufacturers regarding the importance of structured safety monitoring systems poses a significant challenge to market growth
- For instance, in 2023, a widely used imported skin-lightening cream in Southeast Asia was flagged by health authorities after reports of severe irritation and mercury contamination, but delayed reporting by small distributors highlighted the lack of proper cosmetovigilance systems and awareness at the grassroots level
- Underreporting of adverse cosmetic reactions remains a major issue, as many consumers do not formally report side effects, and numerous companies lack standardized systems for capturing and submitting this information to regulatory authorities
- The implementation of robust cosmetovigilance systems requires substantial investment in trained professionals, data management infrastructure, and compliance tools, which can be a financial burden for small brands, start-ups, and manufacturers in developing economies
- In addition, differences in reporting standards and regulatory requirements across countries can complicate global implementation, making it difficult for multinational cosmetic companies to maintain consistent compliance
- Lack of skilled professionals with expertise in cosmetovigilance, toxicology, and regulatory affairs further restricts the effective adoption of structured monitoring processes in some regions
- Overcoming these challenges through industry education, harmonized global standards, affordable compliance solutions, and stronger awareness programs will be essential for the sustained growth and adoption of cosmetovigilance systems worldwide
Cosmetovigilance Market Scope
The market is segmented on the basis of service type, reported categories, and service provider.
- By Service Type
On the basis of service type, the Cosmetovigilance market is segmented into pre-marketing services and post-marketing services. The post-marketing services segment dominated the largest market revenue share of 61.4% in 2025, driven by the growing regulatory requirement to continuously monitor, assess, and report adverse events related to cosmetic products after they are launched in the market. Regulatory bodies in Europe, North America, and parts of Asia strongly emphasize post-market surveillance to ensure ongoing product safety and consumer protection. The increasing number of consumer complaints, allergy incidents, and product recalls is pushing manufacturers to invest more in post-marketing surveillance programs. Moreover, the rapid expansion of online cosmetic sales channels has increased the need for continuous safety tracking across wider populations. Large cosmetic brands are expanding their in-house pharmacovigilance and cosmetovigilance teams to manage risk more effectively. Digital platforms and AI-based reporting systems are further strengthening post-market data collection. Growing consumer awareness about product safety is also contributing to higher reporting rates. These factors collectively reinforce the dominance of the post-marketing services segment in the global market.
The pre-marketing services segment is expected to witness the fastest CAGR of 20.6% from 2026 to 2033, primarily due to the rising number of new product launches in the beauty and personal care industry. Companies are increasingly investing in safety testing, ingredient risk assessment, and regulatory compliance checks even before product commercialization. The rising trend of clean-label cosmetics, organic formulations, and dermatologically-tested claims is encouraging brands to conduct extensive pre-launch evaluations. Start-ups and indie brands are also seeking third-party safety assessments to meet international regulatory standards. Additionally, innovations in in-vitro testing and alternative methods to avoid animal testing are accelerating pre-market service demand. As regulatory scrutiny intensifies globally, pre-marketing safety documentation is becoming mandatory in more regions. This makes the pre-marketing services segment the fastest-growing within the forecast period.
- By Reported Categories
On the basis of reported categories, the Cosmetovigilance market is segmented into skincare, makeup, haircare, perfumes and deodorants, and hair colorants. The skincare segment dominated the largest market revenue share of 38.9% in 2025, owing to its high daily usage and direct, prolonged contact with the skin. The increasing prevalence of skin sensitivities, allergies, acne, and dermatitis cases related to cosmetic usage has driven a higher reporting rate in this category. Skincare products such as creams, lotions, serums, and sunscreens are widely used across all age groups and demographics, further increasing monitoring requirements. The emergence of new active ingredients and chemical combinations also contributes to greater scrutiny. Furthermore, the rapid growth of anti-aging and dermatological skincare segments has expanded the scope of adverse event reporting. Regulatory authorities emphasize intensive monitoring of skincare products due to their high penetration rate. These factors position skincare as the dominant category in the overall cosmetovigilance market.
The hair colorants segment is projected to register the fastest CAGR of 22.3% from 2026 to 2033, driven by the increasing popularity of hair coloring, highlighting, and chemical treatment trends worldwide. Rising fashion consciousness among younger populations and increasing salon visits are significantly boosting the use of permanent and semi-permanent dyes. These products often contain strong chemicals such as ammonia, peroxide, and PPD, which are linked to allergic reactions and scalp damage, increasing the need for stringent monitoring. The rising incidence of reported adverse reactions related to hair dyes is forcing companies to strengthen surveillance mechanisms. Growing regulatory restrictions on certain dye ingredients are also increasing documentation and tracking activities. Additionally, demand for plant-based and ammonia-free alternatives has increased the complexity of safety validation. These combined factors make the hair colorants category the fastest-growing segment.
- By Service Provider
On the basis of service provider, the Cosmetovigilance market is segmented into Clinical Research Organizations (CROs) and Business Process Outsourcing (BPOs). The CROs segment accounted for the largest market revenue share of 57.6% in 2025, owing to their strong scientific expertise, regulatory knowledge, and established infrastructure for safety monitoring. Global cosmetic brands prefer outsourcing their cosmetovigilance activities to CROs because of their experience in adverse event processing, signal detection, and regulatory reporting. CROs also offer integrated solutions including data analysis, risk assessment, and compliance management, which strengthens their market position. Continuous investments in digital reporting platforms and regulatory consulting further enhance their capabilities. The presence of highly trained toxicologists and safety professionals within CROs makes them the preferred choice for complex product portfolios. Increasing collaborations between multinational cosmetic companies and CROs support their dominance in the market.
The BPOs segment is expected to witness the fastest CAGR of 19.4% from 2026 to 2033, driven by the rising demand for cost-efficient and scalable cosmetovigilance solutions. Many mid-sized and emerging cosmetic brands are turning to BPOs for basic services such as data entry, case processing, call center reporting, and consumer complaint management. The availability of skilled but cost-effective labor in developing countries is supporting this trend. Digitalization and cloud-based platforms have also enabled BPOs to handle large data volumes more efficiently. Companies seeking to focus on core product innovation prefer outsourcing administrative safety tasks to BPOs. The increasing number of regional cosmetic brands, especially in Asia-Pacific and Latin America, is accelerating reliance on BPO services. These factors collectively drive the rapid growth of the BPO segment during the forecast period.
Cosmetovigilance Market Regional Analysis
- North America dominated the cosmetovigilance market with the largest revenue share of 43% in 2025, driven by advanced regulatory frameworks, high consumer awareness regarding product safety, the strong presence of leading cosmetic and personal care manufacturers, and a well-established infrastructure for adverse event reporting and compliance monitoring
- This widespread implementation is further supported by increased investments in pharmacovigilance and cosmetovigilance systems, the adoption of digital safety monitoring tools, and rising scrutiny from regulatory bodies, encouraging companies to strengthen post-market surveillance activities across both mass-market and premium product segments
- The U.S. Cosmetovigilance market captured the majority share within North America in 2025, fueled by strict FDA oversight, growing consumer demand for transparency, and the increasing number of product launches in skincare, haircare, and makeup categories, which require continuous monitoring for adverse reactions and long-term safety
U.S. Cosmetovigilance Market Insight
The U.S. cosmetovigilance market is witnessing robust growth due to heightened regulatory enforcement, extensive product consumption, and the adoption of advanced data analytics for tracking and evaluating adverse events. Cosmetic companies are increasingly outsourcing surveillance and reporting functions to specialized service providers to improve efficiency and ensure compliance with evolving guidelines.
Europe Cosmetovigilance Market Insight
The Europe cosmetovigilance market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by strict EU cosmetic regulations, increasing consumer preference for safe and sustainable products, and heightened focus on product traceability. Countries across the region are strengthening their safety monitoring frameworks, leading to greater demand for structured cosmetovigilance programs.
U.K. Cosmetovigilance Market Insight
The U.K. cosmetovigilance market is anticipated to grow at a noteworthy CAGR, supported by independent regulatory oversight following Brexit, growing consumer awareness of ingredient safety, and the rising number of premium and organic product launches requiring extensive post-market monitoring and documentation.
Germany Cosmetovigilance Market Insight
The Germany cosmetovigilance market is expected to expand at a considerable CAGR, fueled by strong research capabilities, a robust cosmetics industry base, and strict adherence to EU safety standards. The country’s focus on innovation, natural formulations, and clinical testing is increasing the demand for advanced safety surveillance services.
Asia-Pacific Cosmetovigilance Market Insight
The Asia-Pacific cosmetovigilance market is poised to grow at the fastest rate during the forecast period, driven by rapid urbanization, an expanding middle-class population, increasing spending on beauty and personal care products, and stronger regulatory focus on product safety in countries such as China, India, Japan, and South Korea.
Japan Cosmetovigilance Market Insight
The Japan cosmetovigilance market is gaining momentum due to high consumer standards, the growing demand for premium and dermatologically tested products, and strong government emphasis on product quality and safety. Companies are increasingly implementing structured reporting systems to maintain compliance and brand trust.
China Cosmetovigilance Market Insight
The China cosmetovigilance market accounted for the largest share within Asia-Pacific in 2025, supported by the country’s massive consumer base, growing awareness of product-related adverse effects, frequent product launches, and the strengthening of cosmetic safety regulations. Domestic and international brands are significantly investing in cosmetovigilance systems to ensure compliance and protect brand reputation.
Cosmetovigilance Market Share
The Cosmetovigilance industry is primarily led by well-established companies, including:
• IQVIA (U.S.)
• Charles River Laboratories (U.S.)
• Eurofins Scientific (Luxembourg)
• TÜV SÜD (Germany)
• Intertek Group plc (U.K.)
• SGS S.A. (Switzerland)
• PRA Health Sciences (U.S.)
• Medpace Holdings, Inc. (U.S.)
• ICON plc (Ireland)
• Accutest Research Laboratories (U.S.)
• ClinWorld Pvt. Ltd. (India)
• Biotrial (France)
• Syneos Health (U.S.)
• WuXi AppTec (China)
• QIMA (Hong Kong)
• Kymos Group (Spain)
• Pharm-Olam International (U.S.)
• ProductLife Group (France)
• INTEGRA (Brazil)
Latest Developments in Global Cosmetovigilance Market
- In December 2022, the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) was enacted in the U.S. under which the U.S. Food and Drug Administration (FDA) gained expanded authority over cosmetic product safety. The law introduced mandatory requirements for adverse-event reporting, facility registration, product listing, and post-market surveillance — significantly increasing compliance obligations for cosmetics companies worldwide
- In December 2023, the FDA issued updated instructions for serious adverse event reporting for cosmetic products, clarifying that responsible persons must report serious undesirable effects within 15 business days, and submit follow-up information if additional medical data emerges within one year — thus reinforcing regulatory commitment to strict cosmetovigilance compliance
- In 2024, new regulatory initiatives were also observed globally: for example, Brazilian Health Regulatory Agency (ANVISA) published a draft regulation (RDC No. 894/2024) outlining “Good Practices in Cosmetovigilance,” scheduled to come into effect in 2025 — signalling a growing emphasis on formalized monitoring and safety protocols in emerging markets
- In June 2025, a leading cosmetovigilance-service provider, AxeRegel AG, expanded its advisory services to include enhanced regulatory-compliance support and cosmetovigilance for cosmetic brands, reflecting rising demand from manufacturers to outsource safety monitoring and adverse-event reporting in view of tightened global regulations
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.