Global Crispr Gene Editing Market
Market Size in USD Billion
CAGR :
% 
 USD
1.83 Billion
USD
14.74 Billion
2024
2032
USD
1.83 Billion
USD
14.74 Billion
2024
2032
| 2025 –2032 | |
| USD 1.83 Billion | |
| USD 14.74 Billion | |
|
|
|
|
CRISPR Gene-Editing Market Size
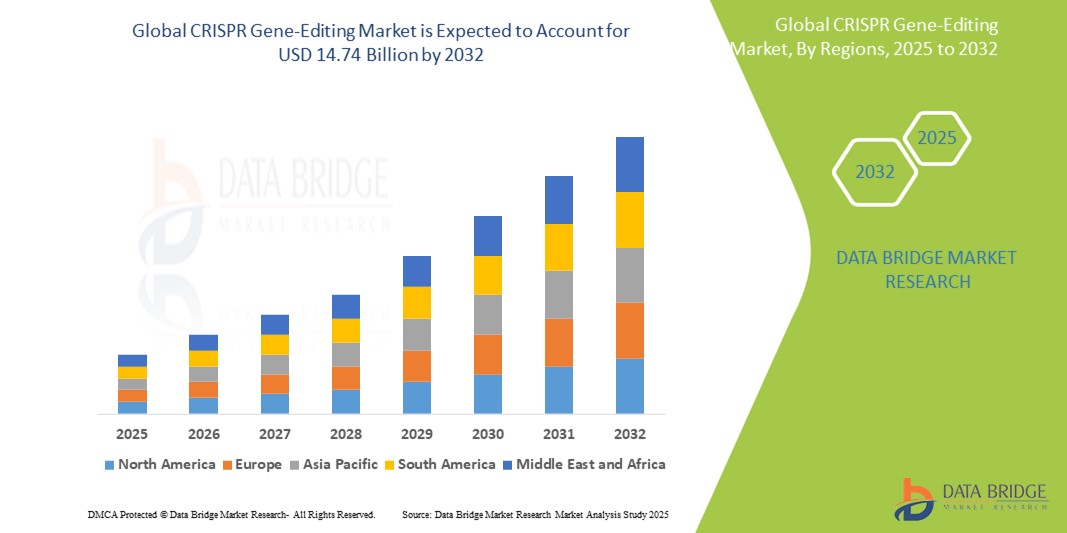
- The global CRISPR gene-editing market size was valued at USD 1.83 billion in 2024 and is expected to reach USD 14.74 billion by 2032, at a CAGR of 29.8% during the forecast period
- The market growth is largely fueled by the rising demand for precise and efficient gene-editing solutions to treat genetic disorders, cancer, and rare diseases. This growing need for targeted therapeutics is driving the adoption of CRISPR gene-editing technologies, thereby accelerating the expansion of the global CRISPR gene-editing market
- Furthermore, continuous advancements in gene-editing research, including the development of next-generation CRISPR systems such as base and prime editing, are improving specificity and reducing off-target effects. These technological innovations are broadening CRISPR’s therapeutic potential and research applications, significantly contributing to the growth of the global CRISPR gene-editing market
CRISPR Gene-Editing Market Analysis
- CRISPR gene-editing technologies, designed to enable precise and efficient modification of DNA, are becoming increasingly integral to therapeutic development, agriculture, and biomedical research due to their versatility, specificity, and transformative potential across multiple sectors
- The growth of the CRISPR gene-editing market is primarily driven by rising investments in genomic medicine, increasing research on rare and genetic disorders, and accelerating clinical trials aimed at developing gene therapies for conditions such as sickle cell disease, cancer, and inherited retinal disorders
- North America dominates the CRISPR gene-editing market with the largest revenue share of 38.5% in 2024, supported by a robust biotechnology ecosystem, strong academic research institutions, favourable regulatory frameworks, and substantial funding for genome-editing innovation and commercialization
- Asia-Pacific is expected to be the fastest growing region in the CRISPR gene-editing market, with an CAGR of 7.2% during the forecast period, propelled by expanding biotechnology capabilities, government-backed genomics initiatives, and a surge in R&D collaborations across countries such as China, Japan, and India
- The CRISPR or Cas9 technology segment dominates the CRISPR gene-editing market, accounting for 59.4% in 2024, owing to its widespread adoption, ease of use, and ongoing advancements that enhance precision, efficiency, and therapeutic safety across clinical and research applications
Report Scope and CRISPR Gene-Editing Market Segmentation
|
Attributes |
CRISPR Gene-Editing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
CRISPR Gene-Editing Market Trends
“Enhanced Precision Through Technological Advancements and Expanding Applications”
- A significant and accelerating trend in the global CRISPR gene-editing market is the growing focus on precision medicine, driven by increasing demand for targeted therapies to treat genetic disorders, cancer, and rare diseases. This shift is compelling biotechnology companies to refine CRISPR tools for greater accuracy, safety, and therapeutic relevance, thereby reshaping the future of gene therapy and molecular diagnostics
- For instance, the development of next-generation CRISPR systems such as base editing and prime editing is seamlessly integrating into genomic research and therapeutic pipelines. These advanced platforms offer the ability to correct mutations without inducing double-strand breaks, significantly reducing the risk of off-target effects and expanding the scope of diseases that can be treated safely and effectively
- Growing awareness among researchers, clinicians, and regulators about the therapeutic potential of CRISPR is accelerating its adoption across various sectors. This awareness is fostering earlier-stage research investments, more rapid clinical translation, and stronger support for regulatory pathways that enable faster, yet safer, market entry for gene-editing therapies
- The increasing prevalence of monogenic disorders and cancers with well-defined genetic mutations is amplifying market demand, as CRISPR-based approaches offer a precise solution to correct the root genetic cause. This clinical need is especially prominent in pediatric and orphan disease segments, where existing therapies are limited or non-existent
- The seamless integration of CRISPR tools into drug discovery, disease modeling, and regenerative medicine workflows is facilitating a more centralized and efficient research ecosystem. Through this convergence, academic institutions, biotech firms, and pharmaceutical companies are forming robust partnerships that accelerate development timelines and maximize therapeutic impact
- This trend toward more intelligent, precise, and clinically validated gene-editing applications is fundamentally reshaping expectations across healthcare and life sciences. Consequently, companies are actively investing in CRISPR technologies with improved delivery methods, regulatory compliance, and scalability, focusing on long-term therapeutic success and broader patient access. The demand for CRISPR solutions that offer curative potential and personalized interventions is growing rapidly, solidifying its role as a transformative force in modern medicine
CRISPR Gene-Editing Market Dynamics
Driver
“Rising Demand for Precision Medicine and Breakthroughs in Genomic Research”
- The growing application of precision medicine, coupled with breakthroughs in genomic sequencing and editing technologies, is a key driver accelerating the global CRISPR gene-editing market. As more conditions are linked to genetic mutations, the ability to directly modify or correct defective genes is transforming therapeutic strategies across oncology, rare diseases, and inherited disorders
- For instance, recent advancements in prime and base editing are expanding the CRISPR toolbox, enabling safer and more accurate interventions for a broader range of diseases. These innovations are reinforcing pharmaceutical and biotech investment, further stimulating clinical trials and research partnerships focused on curative genetic solutions
- As healthcare systems prioritize targeted and minimally invasive therapies, CRISPR gene-editing offers distinct advantages including one-time treatments with long-term benefits, reducing chronic care burdens. This shift is driving both government funding and regulatory support for gene-editing trials
- In addition, the growing number of patients diagnosed with genetic conditions, especially in pediatrics and oncology, has highlighted the limitations of conventional treatments. CRISPR-based therapies provide a viable alternative by addressing the root cause at the genomic level, offering hope for otherwise untreatable conditions
- The combination of increasing public awareness, scientific validation, and expanding access to genomic screening is fostering widespread enthusiasm for CRISPR-based therapeutics. This momentum is being reflected in rising R&D budgets and cross-sector collaborations aimed at scaling development and deployment globally
Restraint/Challenge
“Ethical Uncertainty, Regulatory Hurdles, and High Development Costs”
- Despite its therapeutic potential, the CRISPR gene-editing market faces significant challenges related to ethical considerations and regulatory scrutiny. The prospect of germline editing, for instance, has raised public and institutional concerns regarding the long-term implications of altering heritable traits, delaying progress in certain applications
- In addition, the high development and commercialization costs of CRISPR therapies—driven by complex validation procedures, delivery system innovation, and extensive safety profiling—pose a major barrier to market scalability. These financial demands can discourage smaller biotech firms and limit accessibility in lower-income regions
- Regulatory pathways for CRISPR therapies remain under development in many jurisdictions, creating uncertainty for developers. The absence of harmonized guidelines for gene-editing approval, especially concerning off-target effects and long-term safety, can prolong trial timelines and increase compliance burdens
- Moreover, public skepticism stemming from ethical debates and media portrayals has the potential to influence adoption rates, particularly in markets where scientific literacy or trust in biotechnology is limited
- Addressing these barriers will require strategic investment in education, ethical governance frameworks, and policy advocacy. In addition, enhancing delivery technologies, promoting affordability through biosimilar innovations, and increasing stakeholder transparency will be essential to drive equitable and sustainable growth in the CRISPR gene-editing market
CRISPR Gene-Editing Market Scope
The CRISPR gene-editing market is segmented into multiple notable segments based on therapeutic application, application type, technology, services, products, and end-users.
• By Therapeutic Application
On the basis of therapeutic application, the CRISPR gene-editing market is segmented into oncology and autoimmune or inflammatory diseases. The oncology segment accounted for a dominant market share of 43.2% in 2024, driven by the rising prevalence of cancer and the increasing adoption of CRISPR-based therapies for targeted gene modifications in tumor suppression and immunotherapy.
The autoimmune or inflammatory segment is expected to witness the fastest CAGR from 2025 to 2032, due to advances in gene-editing therapies targeting complex immune-mediated disorders.
• By Application
On the basis of application, the CRISPR gene-editing market is segmented into genome engineering, disease models, functional genomics, and others. Genome engineering held the largest share of 39.5% in 2024, fueled by its broad utility in therapeutic gene correction and synthetic biology.
Disease models is expected to witness the fastest CAGR from 2025 to 2032 as it is critical in drug discovery and understanding genetic diseases.
- By Technology
On the basis of technology, the market is segmented into CRISPR or Cas9, Zinc finger nucleases, and others. The CRISPR or Cas9 segment dominates the largest market revenue share of 59.4% in 2024. This dominance is driven by its high precision, versatility, and ease of use, making it the most widely adopted gene-editing tool across various research and therapeutic applications. Its revolutionary impact on genetic research and disease treatment fuels its market leadership.
The CRISPR or Cas9 segment is also anticipated to witness the fastest growth rate from 2025 to 2032. This rapid growth is fueled by continuous advancements in CRISPR technology, increasing investments in gene therapy research, and the expanding number of clinical trials leveraging CRISPR for treating genetic disorders and cancers.
- By Services
On the basis of services, the market is segmented into Design Tools, Plasmid and Vector, Cas9 and g-RNA, delivery system products, and others. The Cas9 and g-RNA segment (often considered as the core components for CRISPR-based services) held the largest market revenue share in 2024. This is driven by their fundamental role as the key elements required for precise gene editing experiments and therapeutic applications.
The design tools segment is expected to witness the fastest CAGR from 2025 to 2032. This growth is driven by the increasing need for user-friendly, accurate, and automated software and online platforms that facilitate the design of optimal guide RNAs (gRNAs) and CRISPR experiments, enhancing efficiency for researchers and developers.
- By Products
On the basis of products, the market is segmented into GenCrispr or Cas9 kits, GenCrispr Cas9 Antibodies, GenCrispr Cas9 Enzymes, and Others. The GenCrispr or Cas9 kits segment held the largest market revenue share in 2024, driven by the comprehensive and ready-to-use nature of these kits, which provide all necessary reagents and protocols for conducting CRISPR experiments in a standardized and efficient manner. They are essential for both research and diagnostic applications.
The GenCrispr Cas9 Enzymes segment is expected to witness the fastest CAGR from 2025 to 2032, propelled by the increasing demand for high-quality, purified Cas9 and other Cas enzymes that serve as the molecular scissors for precise DNA editing, crucial for advanced gene-editing applications and therapeutic development.
- By End-Users
On the basis of end-users, the market is segmented into biotechnology and pharmaceutical companies, academic and government research institutes, and contract research organizations, and others. The biotechnology and pharmaceutical companies segment accounted for the largest market revenue share, estimated at around 40-45% in 2024. This dominance is attributed to their significant investments in drug discovery, gene therapy development, and the commercialization of gene-edited products.
The academic and government research institutes segment is expected to witness the fastest CAGR from 2025 to 2032. This growth is fueled by increasing government funding for basic and translational research in gene editing, the proliferation of genome research projects, and the vital role these institutes play in exploring novel applications and advancing the foundational science of gene editing technologies.
CRISPR Gene-Editing Market Regional Analysis
- North America dominates the CRISPR gene-editing market with a substantial revenue share of 38.5% in 2024. This leadership is attributed to the region’s advanced healthcare infrastructure, high prevalence of genetic and chronic diseases, and significant investment in biotechnology and pharmaceutical research
- The presence of numerous key industry players, along with strong regulatory frameworks that facilitate the approval and commercialization of innovative gene-editing therapies, further strengthens North America’s market position
- High disposable incomes, growing patient awareness about personalized medicine, and increasing adoption of cutting-edge CRISPR technologies across academic, research, and clinical settings also contribute to robust market growth and widespread utilization in this region
U.S. CRISPR Gene-Editing Market Insight
The U.S. CRISPR gene-editing market captured a dominant revenue share of 80.4% within North America in 2024. This is driven by the high incidence of chronic pruritic and genetic disorders, a strong healthcare infrastructure, and continuous innovation in advanced therapeutics. Patients and healthcare providers prioritize effective, long-lasting solutions such as targeted biologics and gene-editing therapies. A favorable reimbursement environment, significant R&D investment, and active patient advocacy further accelerate market growth.
Europe CRISPR Gene-Editing Market Insight
The Europe CRISPR gene-editing market is projected to grow at a robust CAGR of 5.5% over the forecast period. Growth is primarily driven by increasing cases of chronic skin diseases, aging populations, and a greater focus on improving patient quality of life. Enhanced diagnostics and greater access to specialized care promote the adoption of advanced gene-editing treatments. Europe continues to integrate innovative CRISPR therapies into mainstream healthcare, supporting steady market expansion.
U.K. CRISPR Gene-Editing Market Insight
The U.K. CRISPR gene-editing market is expected to expand at a CAGR of 5.8%, fueled by rising chronic pruritus prevalence and NHS initiatives aimed at improving patient outcomes. Increased awareness among patients and healthcare professionals encourages the use of advanced therapeutic options. The country’s strong pharmaceutical research sector and accessible healthcare infrastructure are key contributors to this growth.
Germany CRISPR Gene-Editing Market Insight
Germany’s CRISPR gene-editing market is forecasted to grow at a CAGR of 5.3%, supported by rising awareness of dermatological disorders and demand for innovative treatments. The country’s healthcare system prioritizes high-quality care and innovation, promoting the uptake of biologics and targeted therapies. Specialist clinics increasingly adopt CRISPR-based solutions, aligning with patient preferences for effective and safe treatments.
Asia-Pacific CRISPR Gene-Editing Market Insight
The Asia-Pacific CRISPR gene-editing market is poised for the fastest growth, with a CAGR of 7.2% from 2025 to 2032. Drivers include increasing disease prevalence, rising disposable incomes, and expanding healthcare infrastructure in countries such as China, Japan, and India. Government initiatives and improved public health awareness are accelerating adoption. Pharmaceutical manufacturing growth in the region also enhances affordability and accessibility.
Japan CRISPR Gene-Editing Market Insight
Japan’s CRISPR gene-editing market is gaining traction with a projected CAGR of 5.9%, driven by an aging population and high healthcare expenditure. There is strong demand for innovative treatments addressing chronic skin conditions such as atopic dermatitis. Advanced therapeutics, including IL-31 receptor antagonists, and robust R&D activities continue to fuel growth across hospital and specialty care sectors.
China CRISPR Gene-Editing Market Insight
China accounted for a significant share of the Asia-Pacific CRISPR gene-editing market in 2024, with around 35% revenue contribution. Rapid urbanization, a growing middle class, and rising dermatological disease burden propel market expansion. Domestic biopharmaceutical advancements and increasing access to innovative therapies support China’s prominent role in the regional market growth.
CRISPR Gene-Editing Market Share
The CRISPR gene-editing industry is primarily led by well-established companies, including:
- Applied StemCell (U.S.)
- LUMITOS AG (U.S.)
- Synthego (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- GenScript (China)
- Addgene (U.S.)
- Merck KGaA (Germany)
- Intellia Therapeutics, Inc. (U.S.)
- Cellectis (France)
- Precision Biosciences (U.S.)
- Caribou Biosciences, Inc. (U.S.)
- OriGene Technologies, Inc. (U.S.)
- Novartis AG (Switzerland)
- New England Biolabs (U.S.)
- ROCKLAND IMMUNOCHEMICALS INC. (U.S.)
- ToolGen, Inc. (South Korea)
- TAKARA BIO INC. (Japan)
- Agilent Technologies, Inc. (U.S.)
- Abcam Limited (U.K.)
- CRISPR Therapeutics AG (Switzerland)
Latest Developments in Global CRISPR Gene-Editing Market
- In January 2024, CRISPR Therapeutics AG and Vertex Pharmaceuticals announced the U.S. FDA approval of Casgevy (exagamglogene autotemcel), marking the first-ever approval of a CRISPR-based gene-editing therapy for the treatment of sickle cell disease and transfusion-dependent β-thalassemia. This milestone signifies a transformative breakthrough in genetic medicine, offering a one-time curative approach for patients with severe genetic blood disorders
- In March 2024, Intellia Therapeutics and its partner Regeneron Pharmaceuticals reported promising Phase 1 clinical trial data for NTLA-2002, a CRISPR/Cas9-based in vivo gene-editing therapy aimed at treating hereditary angioedema (HAE). The early data revealed substantial reductions in HAE attacks, suggesting a potential for durable, single-dose treatment in a traditionally underserved therapeutic area
- In April 2024, Editas Medicine announced successful in vivo preclinical results of its CRISPR-edited cell therapy for rhabdomyosarcoma, a rare and aggressive pediatric cancer. The company’s lead candidate demonstrated targeted tumor regression and minimal off-target effects, advancing Editas’ oncology pipeline toward clinical trials
- In February 2024, Synthego, a U.S.-based genome engineering company, launched its Automated Cell Engineering Platform, which leverages AI and CRISPR to streamline and scale gene-editing workflows for both research and therapeutic development. This innovation aims to reduce CRISPR experiment timelines by up to 50%, accelerating the path to discovery and commercialization
- In November 2023, Beam Therapeutics received IND (Investigational New Drug) clearance from the U.S. FDA for BEAM-101, a base-editing CRISPR therapy for the treatment of sickle cell disease. Unlike traditional CRISPR approaches, BEAM-101 uses base editing technology to mimic a naturally occurring genetic variant associated with reduced disease severity, reflecting a more refined and potentially safer gene-editing strategy
- In December 2023, Caribou Biosciences reported clinical trial progress for CB-010, its lead CAR-T cell therapy candidate engineered using CRISPR genome editing. Early Phase 1 results indicated durable anti-tumor activity in patients with relapsed/refractory B cell non-Hodgkin lymphoma. This marked one of the first allogeneic CAR-T therapies to enter clinical trials using CRISPR to enhance cell persistence and reduce immune rejection
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.