Global Cronobacter Market
Market Size in USD Billion
CAGR :
% 
 USD
1.00 Billion
USD
1.43 Billion
2025
2033
USD
1.00 Billion
USD
1.43 Billion
2025
2033
| 2026 –2033 | |
| USD 1.00 Billion | |
| USD 1.43 Billion | |
|
|
|
|
Cronobacter Market Size
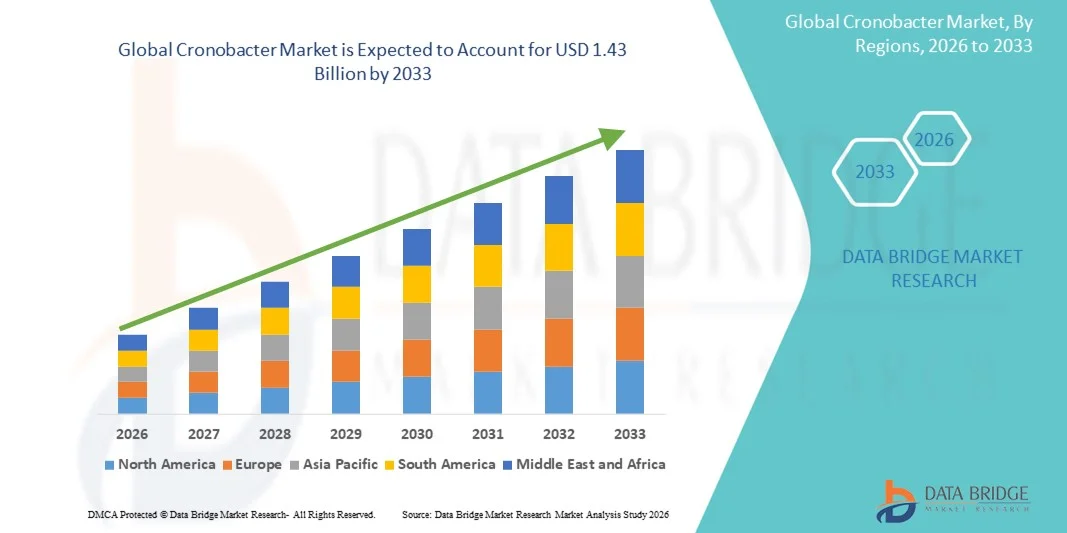
- The global Cronobacter market size was valued at USD 1.00 billion in 2025 and is expected to reach USD 1.43 billion by 2033, at a CAGR of 4.60% during the forecast period
- The market growth is largely fueled by increasing awareness of foodborne pathogens, the rising prevalence of neonatal infections, and the growing need for food safety and quality control across infant formula and powdered milk industries
- Furthermore, stringent regulatory frameworks and proactive initiatives by healthcare and food safety authorities to monitor and control Cronobacter contamination are driving demand for rapid detection and prevention solutions. These converging factors are accelerating the adoption of advanced Cronobacter testing and mitigation strategies, thereby significantly boosting the industry's growth
Cronobacter Market Analysis
- Cronobacter, a genus of pathogenic bacteria, is primarily associated with severe infections in neonates and infants, including sepsis and meningitis, making early detection, treatment, and prevention critical in both clinical and food safety settings
- The rising demand for effective Cronobacter management is driven by increasing awareness of infant food safety, stringent regulatory standards for powdered infant formula, and growing investments in rapid diagnostic and treatment solutions
- North America dominated the Cronobacter market with the largest revenue share of 37.2% in 2025, attributed to advanced healthcare infrastructure, stringent food safety regulations, and high adoption of diagnostic technologies, with the U.S. witnessing notable growth in hospital-based testing and antibiotic treatment protocols supported by both established pharmaceutical companies and specialized diagnostics firms
- Asia-Pacific is expected to be the fastest growing region in the Cronobacter market during the forecast period due to expanding neonatal healthcare facilities, increasing awareness of foodborne infections, and rising investments in diagnostic laboratories and hospital networks
- Antibiotic segment dominated the Cronobacter treatment market with a market share of 45.4% in 2025, driven by its established efficacy, widespread clinical adoption, and continuous development of targeted therapies to combat antibiotic-resistant strains
Report Scope and Cronobacter Market Segmentation
|
Attributes |
Cronobacter Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cronobacter Market Trends
Advancements in Rapid Detection and Diagnostic Technologies
- A major and accelerating trend in the global Cronobacter market is the development of rapid, sensitive, and accurate diagnostic tools for early detection in neonatal and infant care settings, reducing the risk of severe infections
- For instance, PCR-based test kits now allow detection of Cronobacter in powdered infant formula within hours, significantly improving response times compared to traditional culture methods
- Integration of biosensors and microfluidic platforms enables point-of-care testing, offering faster, real-time results and minimizing hospital laboratory dependence
- Advanced diagnostic platforms facilitate centralized monitoring of infection outbreaks in hospitals and production facilities, allowing for timely interventions and preventive actions
- Development of AI and machine learning-based predictive models is emerging to identify potential contamination sources and forecast outbreak risks in production and clinical environments
- Novel packaging and sterilization technologies are being integrated with diagnostic monitoring to enhance food safety and reduce Cronobacter prevalence in infant nutrition
- This trend towards rapid, precise, and accessible detection technologies is reshaping expectations for infant food safety and clinical diagnostics
- The demand for innovative Cronobacter diagnostic solutions is rising across both healthcare and food manufacturing sectors, as stakeholders increasingly prioritize prevention, safety, and early intervention
Cronobacter Market Dynamics
Driver
Increasing Awareness of Infant Food Safety and Regulatory Compliance
- The rising awareness among parents, healthcare providers, and manufacturers regarding infant food safety is a key driver for the growing demand for Cronobacter detection and prevention solutions
- For instance, in March 2025, Nestlé expanded its neonatal formula safety program by implementing enhanced Cronobacter testing protocols in multiple production facilities
- Strict regulatory standards and guidelines by organizations such as the FDA and WHO are prompting manufacturers to adopt advanced detection and monitoring solutions to prevent contamination
- As concerns over neonatal infections rise, healthcare institutions are increasingly investing in hospital-based testing, rapid diagnostic kits, and staff training programs to minimize infection risks
- Growing demand for powdered infant formula and ready-to-feed products globally is pushing manufacturers to implement rigorous quality control measures, further boosting market growth
- Enhanced focus on preventive measures, early diagnosis, and food safety compliance is propelling the adoption of Cronobacter monitoring and treatment solutions across the healthcare and food sectors
- Increased collaborations between diagnostic technology providers and infant formula manufacturers are creating opportunities for integrated safety solutions across the supply chain
- Rising public and private funding for research on neonatal infections and Cronobacter epidemiology is driving innovation and accelerating market expansion
Restraint/Challenge
High Testing Costs and Technical Complexity
- The relatively high cost of advanced diagnostic equipment and laboratory testing procedures limits accessibility, particularly in developing countries, posing a significant challenge to market expansion
- For instance, small-scale formula manufacturers often face budget constraints in implementing comprehensive Cronobacter testing programs, slowing adoption rates
- Technical complexity and the need for trained personnel to operate sophisticated detection systems can impede widespread implementation in hospitals and production facilities
- Variability in laboratory infrastructure and lack of standardized protocols in certain regions hinder consistent and reliable Cronobacter monitoring
- While rapid diagnostic solutions are becoming more efficient, ongoing maintenance, calibration, and quality assurance requirements add to operational costs, limiting uptake
- Limited awareness among smaller healthcare facilities and regional manufacturers about the latest detection technologies can delay adoption and compromise infant safety
- Evolving regulations across different regions require continuous updates to testing protocols, adding regulatory compliance burdens for manufacturers and laboratories
- Overcoming these challenges through cost-effective testing solutions, simplified diagnostic platforms, and training programs will be critical for sustained market growth
Cronobacter Market Scope
The market is segmented on the basis of treatment, diagnosis, dosage, route of administration, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the Cronobacter market is segmented into antibiotic and others. The antibiotic segment dominated the market with the largest revenue share of 45.4% in 2025, driven by its established efficacy in treating severe neonatal infections such as sepsis and meningitis. Hospitals and neonatal care units widely prefer antibiotics due to their rapid action and reliability in clinical outcomes. Continuous development of targeted antibiotic therapies to combat resistant Cronobacter strains is further reinforcing dominance. The segment also benefits from established clinical guidelines recommending antibiotics as the primary treatment, making it a standard protocol in neonatal intensive care units (NICUs). In addition, pharmaceutical companies’ ongoing research and development in broad-spectrum antibiotics ensures a steady supply and adoption across key markets.
The others segment is anticipated to witness the fastest growth rate during the forecast period due to increasing adoption of complementary therapies such as probiotics, immunoglobulins, and supportive care solutions. These alternatives are gaining traction in preventive care for at-risk neonates and as adjunct therapies to reduce antibiotic dependence. Growing awareness among healthcare professionals about the benefits of combination treatment approaches is fueling demand in hospitals and specialty clinics. Moreover, emerging research highlighting the potential of microbiome-modulating interventions to prevent Cronobacter colonization is supporting this growth.
- By Diagnosis
On the basis of diagnosis, the market is segmented into blood tests, laboratory tests, and others. The laboratory tests segment dominated the market in 2025, driven by its accuracy and wide use in hospital laboratories and food testing facilities. Conventional culture methods and molecular techniques such as PCR are widely relied upon for confirming Cronobacter infections in neonates and contaminated infant formula. Laboratories with established protocols ensure regulatory compliance and quality assurance, making this segment the preferred choice. Strong partnerships between diagnostic kit manufacturers and clinical labs have further reinforced the segment’s market share. In addition, laboratory tests are essential for epidemiological studies and outbreak monitoring, enhancing their relevance in public health surveillance.
The blood tests segment is expected to witness the fastest growth during the forecast period, fueled by the rising adoption of rapid, point-of-care diagnostic technologies. Blood-based detection allows early identification of systemic Cronobacter infections, enabling prompt treatment. Advancements in biosensors and microfluidic devices are making these tests faster, less invasive, and more accurate. Increasing investments in neonatal healthcare infrastructure and hospital-based diagnostic solutions are further driving adoption. Blood tests also provide critical data for monitoring treatment effectiveness, further contributing to their growing demand.
- By Dosage
On the basis of dosage, the market is segmented into tablet, injection, and others. The injection segment dominated the market in 2025 due to its direct and rapid delivery of antibiotics in critical neonatal infections, ensuring immediate therapeutic efficacy. Intravenous administration in NICUs is preferred for severe cases of sepsis or meningitis, where oral delivery may be impractical. The segment is supported by well-established clinical protocols and physician familiarity, making it the standard of care in hospitals. Pharmaceutical advancements in injection formulations enhance stability and dosage accuracy, further consolidating dominance. Injections also allow combination therapies to be administered efficiently in acute care settings.
The tablet segment is expected to witness the fastest growth during the forecast period due to rising outpatient treatments and supportive care initiatives in neonatal and pediatric healthcare. Tablets offer convenience, cost-effectiveness, and ease of administration in non-critical settings or follow-up treatments after hospital discharge. Increasing awareness about antimicrobial stewardship and dosage compliance is boosting the adoption of oral therapies in neonatal care management.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral, intravenous, and others. The intravenous segment dominated the market in 2025 owing to its critical role in delivering life-saving antibiotics directly into the bloodstream for severely ill neonates. IV administration ensures rapid therapeutic effect and precise dosing, critical in NICUs for infants with systemic Cronobacter infections. Hospitals prefer IV solutions due to established safety protocols and proven efficacy in acute care scenarios. Technological advancements in infusion systems and pediatric IV formulations support widespread use and adoption. Moreover, IV administration facilitates co-administration with other supportive therapies, reinforcing its market dominance.
The oral segment is expected to witness the fastest growth rate during the forecast period during the forecast period as demand rises for outpatient care and preventive treatment options. Oral administration is preferred for follow-up therapy post-hospitalization and for mild or controlled cases. Easy-to-administer oral solutions and tablets for infants and children are boosting adoption in home care settings. Growing parental awareness and hospital discharge programs promoting oral antibiotics for ongoing therapy are contributing to the segment’s expansion.
- By End-Users
On the basis of end-users, the market is segmented into clinics, hospitals, and others. The hospital segment dominated the market in 2025 due to the concentration of NICUs and advanced neonatal care facilities capable of managing severe Cronobacter infections. Hospitals have access to trained personnel, diagnostic laboratories, and approved antibiotic therapies, ensuring effective management of infections. Institutional protocols for neonatal sepsis prevention and food safety testing further reinforce hospital dominance. Collaboration with pharmaceutical companies and diagnostic kit providers supports seamless procurement and adoption of treatments. The segment also benefits from centralized infection monitoring and outbreak control initiatives.
The clinics segment is expected to witness the fastest growth during the forecast period, driven by increasing demand for outpatient monitoring, preventive care, and early detection services. Smaller healthcare facilities are adopting rapid diagnostic tools and treatment protocols to manage mild or early-stage infections before escalation. Growing awareness among pediatricians and parents about Cronobacter risks encourages clinic visits for preventive screening and care. Telemedicine and portable testing solutions are further boosting the adoption of clinic-based services.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market in 2025 due to direct access to NICUs and centralized procurement systems, ensuring timely availability of antibiotics and diagnostic kits. Hospital pharmacies provide controlled dispensing, compliance with medical protocols, and integration with patient care programs. Collaborations with pharmaceutical manufacturers and diagnostic companies ensure a reliable supply chain. The segment also benefits from bulk procurement advantages, making it cost-efficient for hospitals managing neonatal care.
The online pharmacy segment is expected to witness the fastest growth rate during the forecast period, driven by increasing e-commerce adoption and demand for home delivery of preventive therapies and supportive care medications. Online platforms allow parents and caregivers to access required treatments conveniently, especially for follow-up care. Integration of telemedicine services with online pharmacies is further accelerating adoption. Rising awareness about convenience, affordability, and accessibility is fueling the growth of online distribution channels.
Cronobacter Market Regional Analysis
- North America dominated the Cronobacter market with the largest revenue share of 37.2% in 2025, attributed to advanced healthcare infrastructure, stringent food safety regulations, and high adoption of diagnostic technologies
- Hospitals and neonatal care units in the region prioritize early detection and effective treatment of Cronobacter infections, investing in advanced diagnostic tools, rapid testing kits, and hospital-based monitoring programs to safeguard infant health
- This widespread adoption is further supported by high healthcare expenditure, a strong regulatory framework from agencies such as the FDA and CDC, and proactive measures by infant formula manufacturers, establishing Cronobacter detection and treatment solutions as a preferred standard in both clinical and food safety settings
U.S. Cronobacter Market Insight
The U.S. Cronobacter market captured the largest revenue share of 82% in 2025 within North America, fueled by advanced neonatal healthcare infrastructure and strict regulatory oversight of infant food safety. Hospitals and neonatal care units are increasingly prioritizing rapid detection and effective treatment of Cronobacter infections in infants. The growing adoption of point-of-care diagnostics, hospital-based monitoring programs, and antibiotic treatment protocols further propels the market. Moreover, the presence of leading pharmaceutical and diagnostic companies, combined with strong public awareness campaigns on infant nutrition safety, is significantly contributing to market expansion.
Europe Cronobacter Market Insight
The Europe Cronobacter market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent food safety regulations and rising awareness of neonatal infections. Increasing urbanization and the demand for safe infant nutrition are fostering the adoption of advanced diagnostic and treatment solutions. European hospitals and neonatal care facilities are emphasizing preventive measures, including routine testing of powdered infant formula and early intervention treatments. The region is witnessing significant growth across hospital and clinic applications, with Cronobacter management being integrated into both new healthcare protocols and existing pediatric care frameworks.
U.K. Cronobacter Market Insight
The U.K. Cronobacter market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising parental awareness of infant food safety and the implementation of stringent regulatory standards. Concerns regarding neonatal infections and hospital-acquired infections are encouraging healthcare providers to adopt rapid detection and treatment solutions. The U.K.’s emphasis on quality healthcare, along with its robust diagnostic and hospital infrastructure, is expected to continue stimulating market growth. Adoption of point-of-care testing and advanced antibiotic therapies further reinforces the country’s proactive approach to managing Cronobacter risks.
Germany Cronobacter Market Insight
The Germany Cronobacter market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of neonatal infections and stringent food safety compliance requirements. Germany’s well-developed healthcare system, combined with its focus on research and innovation in neonatal care, promotes the adoption of advanced diagnostics and antibiotic treatments. Hospitals and neonatal intensive care units are emphasizing preventive protocols and rapid outbreak response measures. Furthermore, collaborations between diagnostic providers and formula manufacturers are enhancing Cronobacter management, aligning with local consumer expectations for safety and reliability.
Asia-Pacific Cronobacter Market Insight
The Asia-Pacific Cronobacter market is poised to grow at the fastest CAGR of 25% during the forecast period of 2026 to 2033, driven by increasing neonatal healthcare facilities, rising disposable incomes, and growing awareness of infant food safety in countries such as China, Japan, and India. Government initiatives promoting infant nutrition safety, coupled with investments in hospital infrastructure and diagnostic laboratories, are driving the adoption of Cronobacter detection and treatment solutions. In addition, as APAC becomes a hub for diagnostic kit production, the affordability and accessibility of testing and treatment options are expanding to a wider population, supporting rapid market growth.
Japan Cronobacter Market Insight
The Japan Cronobacter market is gaining momentum due to the country’s advanced healthcare infrastructure, high awareness of neonatal infection risks, and emphasis on infant nutrition safety. Hospitals and neonatal units are increasingly adopting rapid detection methods and antibiotic treatment protocols to prevent severe infections. Integration of advanced diagnostic technologies in clinical workflows and government-driven food safety programs is fueling growth. Moreover, Japan’s aging population and focus on high-quality neonatal care are expected to sustain demand for efficient Cronobacter management solutions in both residential and hospital settings.
India Cronobacter Market Insight
The India Cronobacter market accounted for the largest market revenue share in Asia Pacific in 2025, attributed to the country’s expanding healthcare facilities, rapid urbanization, and rising awareness of infant food safety. India is witnessing increasing adoption of hospital-based monitoring programs, rapid diagnostic kits, and preventive measures in neonatal care units. Government initiatives promoting child nutrition and safety, along with availability of cost-effective diagnostic and treatment solutions, are key factors propelling the market. Strong domestic diagnostic manufacturers and growing parental awareness of infection risks further support the market’s rapid expansion across both hospital and clinic settings.
Cronobacter Market Share
The Cronobacter industry is primarily led by well-established companies, including:
- Hygiena, LLC (U.S.)
- BIOMÉRIEUX (France)
- Thermo Fisher Scientific Inc. (U.S.)
- Neogen Corporation (U.S.)
- 3M (U.S.)
- Bio Rad Laboratories, Inc. (U.S.)
- R Biopharm AG (Germany)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer. (U.S.)
- QIAGEN (Netherlands)
- Romer Labs Division Holding GmbH (Austria)
- Eurofins Scientific SE (Luxembourg)
- Merck KGaA (Germany)
- Charm Sciences, Inc. (U.S.)
- ALS Limited (Australia)
- Danaher (U.S.)
- Shimadzu Corporation (Japan)
- Genetic ID NA, Inc. (U.S.)
- Mérieux NutriSciences (France)
- Biocontrol Systems, Inc. (U.S.)
What are the Recent Developments in Global Cronobacter Market?
- In September 2023, The FDA published a formal strategy to prevent Cronobacter sakazakii illnesses associated with powdered infant formula, announcing measures to improve manufacturing oversight, strengthen regulatory inspections (with a proposed “Office of Critical Foods”), and enhance consumer safety communications
- In August 2023, The FDA issued warning letters to three major infant‑formula makers ByHeart Inc., Reckitt/Mead Johnson Nutrition, and Perrigo Wisconsin, LLC citing violations of infant formula manufacturing regulations after inspections in their facilities, specifically pointing to failures in pathogen control
- In February 2023, Reckitt (Mead Johnson) voluntarily recalled around 145,000 cans of its Enfamil ProSobee Simply Plant‑Based Infant Formula due to a possible cross‑contamination with Cronobacter sakazakii; the batches were later found to test negative, but the company traced the issue to a third‑party supplier
- In September 2022, The CDC closed its outbreak investigation into four infant Cronobacter cases linked to the Sturgis facility, noting that while there were 4 confirmed cases (2 deaths), genomic testing showed the clinical isolates did not closely match the environmental strains identified at Abbott
- In February 2022, The FDA launched an investigation into Cronobacter sakazakii infections linked to powdered infant formula produced at Abbott Nutrition’s Sturgis, Michigan facility. The inspection uncovered five different strains of Cronobacter in environmental swabs, raising serious concerns about production hygiene
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.