Global Cryopreserved Fat Graft Viability Enhancement Solutions Market
Market Size in USD Million
CAGR :
% 
 USD
95.00 Million
USD
266.84 Million
2024
2032
USD
95.00 Million
USD
266.84 Million
2024
2032
| 2025 –2032 | |
| USD 95.00 Million | |
| USD 266.84 Million | |
|
|
|
|
Cryopreserved Fat Graft Viability Enhancement Solutions Market Size
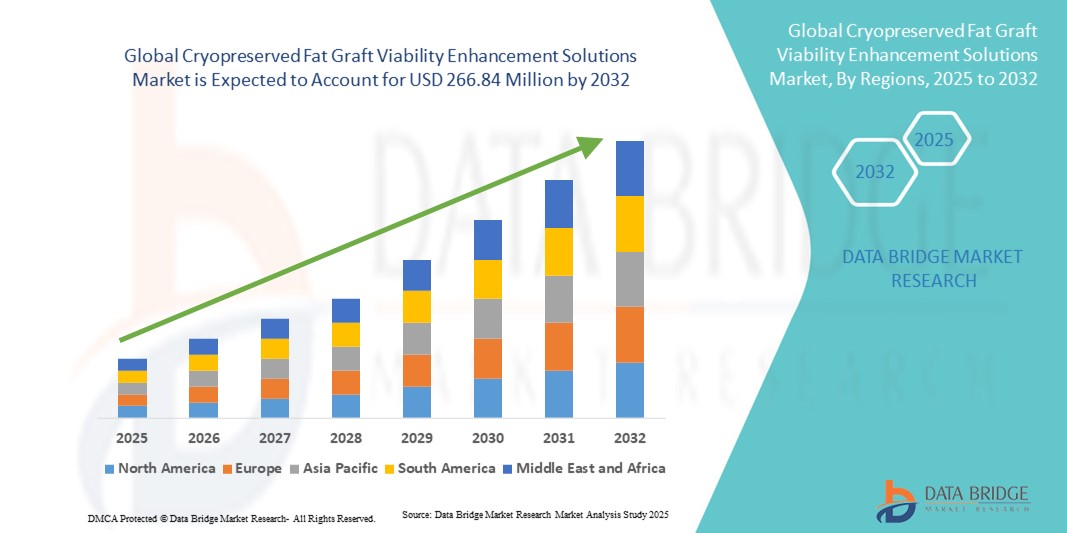
- The global cryopreserved fat graft viability enhancement solutions market size was valued at USD billion in 2024 and is expected to reach USD 266.84 million by 2032, at a CAGR of 13.78% during the forecast period
- The market growth is largely fueled by the rising demand for fat grafting procedures in aesthetic and reconstructive surgery, coupled with advancements in cryopreservation technologies that improve adipocyte and stem cell survival post-thaw
- Furthermore, increasing adoption of bioactive enhancers such as platelet-rich plasma, stem-cell–enriched grafts, and extracellular vesicles is establishing cryopreserved grafts as a reliable, long-term option for staged procedures. These converging factors are accelerating clinical uptake, thereby significantly boosting the industry’s growth
Cryopreserved Fat Graft Viability Enhancement Solutions Market Analysis
- Cryopreserved fat graft viability enhancement solutions, designed to preserve adipocyte and stem cell function during freezing and thawing, are increasingly vital in both aesthetic and reconstructive surgery due to their ability to support repeat procedures, extend graft availability, and improve long-term clinical outcomes
- The escalating demand for these solutions is primarily fueled by the rising number of cosmetic and reconstructive fat grafting procedures, advancements in cryoprotectant formulations and bioactive enhancers, and a growing preference for natural, autologous tissue over synthetic fillers
- North America dominated the cryopreserved fat graft viability enhancement solutions market with the largest revenue share of 39.2% in 2024, supported by high volumes of cosmetic surgery, advanced clinical infrastructure, and strong adoption of cryobanking services, with the U.S. leading in translational research and clinical implementation of adipose preservation protocols
- Asia-Pacific is expected to be the fastest growing region during the forecast period due to expanding aesthetic tourism, rising disposable incomes, and the growing adoption of regenerative medicine practices across countries such as South Korea, China, and India
- Cryopreservation media and cryoprotectants segment dominated the market with a share of 42% in 2024, driven by their critical role in maintaining adipocyte and stromal vascular fraction viability, and their established use as the foundation of current clinical cryopreservation protocols
Report Scope and Cryopreserved Fat Graft Viability Enhancement Solutions Market Segmentation
|
Attributes |
Cryopreserved Fat Graft Viability Enhancement Solutions Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cryopreserved Fat Graft Viability Enhancement Solutions Market Trends
Integration of Bioactive Enhancers to Improve Graft Survival
- A significant and accelerating trend in the global cryopreserved fat graft viability enhancement solutions market is the integration of bioactive enhancers such as platelet-rich plasma (PRP), stromal vascular fraction (SVF), and extracellular vesicles to improve graft retention and angiogenesis
- For instance, PRP-enriched cryopreserved grafts are being increasingly adopted in aesthetic clinics to promote faster vascularization and long-term fat survival. Similarly, SVF supplementation is used in reconstructive procedures to enhance cell viability and regenerative outcomes
- Bioactive enhancers allow solutions to provide improved cellular metabolism post-thaw, reduce apoptosis, and extend graft longevity. For instance, ASC-derived extracellular vesicles have been tested in preclinical models to significantly improve angiogenesis and healing capacity after fat grafting
- The integration of such biologically active agents into cryopreservation protocols is facilitating multi-stage reconstructive and cosmetic procedures where repeat grafting is required. Through a single preservation cycle, clinicians can access viable adipose tissue enhanced with supportive factors, increasing procedural efficiency
- This trend towards biologically enriched cryopreservation is reshaping expectations for fat grafting by aligning outcomes with regenerative medicine standards. Consequently, companies and research groups are focusing on developing GMP-compliant cryopreservation kits with integrated enhancers to offer superior clinical reliability
- The demand for bioactive-enhanced cryopreserved fat graft solutions is growing rapidly across both aesthetic and reconstructive sectors, as patients increasingly prioritize natural results, improved graft take rates, and reduced procedure frequency
Cryopreserved Fat Graft Viability Enhancement Solutions Market Dynamics
Driver
Rising Cosmetic and Reconstructive Fat Grafting Procedures
- The increasing number of aesthetic procedures such as facial contouring and breast augmentation, combined with reconstructive applications after cancer surgery or trauma, is a significant driver for the heightened demand for cryopreserved fat graft viability enhancement solutions
- For instance, in 2024 multiple research collaborations reported advances in cryoprotectant formulations and cryobanking services, ensuring high adipocyte and stem cell survival rates, which are expected to accelerate clinical adoption worldwide
- As patients and surgeons seek more predictable and durable outcomes, cryopreservation allows long-term storage of autologous fat, reducing the need for repeated liposuction procedures and enabling staged surgeries with higher convenience
- Furthermore, the growing popularity of regenerative medicine and stem cell–based therapies is positioning adipose-derived stem cells from cryopreserved grafts as a vital resource, boosting the clinical and research demand for optimized preservation solutions
- The convenience of storing viable adipose tissue, enabling repeat procedures without new harvests, and the ability to enrich grafts with bioactive agents are key factors propelling the adoption of these solutions in both aesthetic and reconstructive markets. The trend towards cryobanking services and clinical-grade preservation kits further contributes to market growth
Restraint/Challenge
Cell Viability Loss and Regulatory Compliance Hurdle
- Concerns surrounding post-thaw adipocyte viability and variability in stromal vascular fraction recovery pose a significant challenge to broader market penetration. As outcomes can be inconsistent without optimized cryoprotectant formulations, clinicians remain cautious about routine use
- For instance, published studies have shown that simple freezing at –20°C results in poor cell survival, making controlled-rate freezing and validated solutions necessary, yet not universally available across all clinics
- Addressing these concerns through advanced cryoprotectants, validated storage protocols, and standardized thawing techniques is crucial for building surgeon confidence. Companies and academic groups emphasize their proprietary cryoprotectant blends and cell recovery rates to reassure clinical users
- In addition, the relatively high initial cost of GMP-compliant cryopreservation kits and long-term cryostorage services can be a barrier to adoption for smaller clinics and patients in cost-sensitive regions. While simplified short-term clinic storage is more affordable, premium solutions remain priced higher
- While costs are gradually decreasing with broader adoption, the perceived premium of cryopreserved grafting compared to traditional fresh grafting can still hinder widespread uptake, particularly among aesthetic patients prioritizing affordability
- Overcoming these challenges through enhanced cryopreservation media, clinician training, and the development of cost-effective, ready-to-use kits will be vital for sustained market growth
Cryopreserved Fat Graft Viability Enhancement Solutions Market Scope
The market is segmented on the basis of product type, technology, storage method, end user, and distribution channel.
- By Product Type
On the basis of product type, the market is segmented into cryopreservation media & cryoprotectants, turnkey cryopreservation kits, additive/bioactive enhancer solutions, and storage & handling consumables. The cryopreservation media & cryoprotectants segment dominated the market with the largest revenue share of 42% in 2024, driven by its indispensable role in maintaining adipocyte viability and preventing ice crystal damage during freezing. These solutions are widely used in both clinical and research settings, making them the backbone of fat graft preservation. Their well-established efficacy and broad distribution ensure consistent demand. Moreover, the availability of optimized formulations and continuous refinements have reinforced their dominance as the standard solution for most end users.
The turnkey cryopreservation kits segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by their ability to offer ready-to-use, standardized, and regulatory-friendly preservation solutions. Clinics and ambulatory surgery centers increasingly prefer kits as they reduce procedural variability and save time for healthcare staff. The convenience of prepackaged components, including media, cryovials, and validated protocols, appeals to users seeking efficiency and reliability. Rising adoption among aesthetic surgeons in developed and emerging regions likely is expected to accelerate this growth. With growing preference for simplified workflows, these kits are positioned to achieve the highest CAGR during the forecast period.
- By Technology
On the basis of technology, the market is segmented into controlled-rate freezing, vitrification, novel cryoprotectant chemistries, and hybrid short-term preservation protocols. The controlled-rate freezing segment dominated the market with the largest share in 2024, as it remains the most validated and widely accepted preservation technique. This technology minimizes cellular stress during cooling, thereby preserving adipocyte viability and improving graft retention rates post-thaw. Hospitals and biobanks rely on it due to its proven reliability, scalability, and compatibility with existing infrastructure. Its adoption across both clinical and academic institutions further consolidates its dominant market position.
The novel cryoprotectant chemistries segment is projected to grow at the fastest CAGR from 2025 to 2032, driven by increasing innovation in less-toxic, antioxidant-based, and apoptosis-inhibiting formulations. These new solutions are being developed to replace traditional DMSO-based media, addressing toxicity and compliance concerns while enhancing long-term viability. Clinical studies are demonstrating superior graft survival and patient outcomes with these novel approaches. Investment in translational research and biotechnology partnerships is accelerating their commercialization. As awareness spreads and regulatory approvals expand, these chemistries are set to achieve the strongest growth momentum in the coming years.
- By Storage Method
On the basis of storage method, the market is segmented into liquid nitrogen vapor storage (≤ –150°C), ultra-low freezer storage (–80°C), short-term clinic-based cryopreservation, and long-term biobank cryostorage. The liquid nitrogen vapor storage segment dominated the market in 2024, owing to its unmatched ability to ensure long-term preservation and maintain high cell viability. It has long been considered the gold standard in biobanking due to its proven efficacy in preventing structural and metabolic damage. Its established use across hospitals, biobanks, and research institutes continues to reinforce its dominance. The infrastructure-intensive nature of this method has not slowed its adoption, as institutions prioritize quality and long-term reliability.
The short-term clinic-based cryopreservation segment is expected to register the fastest growth from 2025 to 2032, driven by the rising need for immediate, flexible storage solutions within aesthetic clinics. These methods provide convenient in-house preservation for repeat procedures without reliance on third-party biobanks. Clinics in emerging regions are particularly adopting these systems to offer affordable, accessible fat graft storage. The increasing availability of compact and cost-effective freezing devices is further supporting adoption. As repeat demand for cosmetic enhancements rises, short-term clinic-based storage is positioned to expand rapidly as the preferred choice for immediate application.
- By End User
On the basis of end user, the market is segmented into plastic & aesthetic surgery clinics, ambulatory surgery centers, hospitals, biobanks & cryopreservation service providers, and research & academic institutes. The plastic & aesthetic surgery clinics segment dominated the market with the largest share in 2024, reflecting the high procedural volume of cosmetic fat grafting worldwide. Clinics remain the primary point of care for patients seeking breast augmentation, facial rejuvenation, and reconstructive procedures, where graft viability directly influences outcomes. Their repeat usage of consumables and kits drives consistent revenue. The growing popularity of minimally invasive aesthetics continues to strengthen the leadership of this segment.
The biobanks & cryopreservation service providers segment is anticipated to grow at the fastest rate during the forecast period, as patient demand for long-term adipose tissue storage continues to rise. These institutions provide specialized infrastructure and services that many clinics cannot afford in-house, making them highly attractive for outsourcing. Expansion of commercial fat banking services in regions such as North America, Europe, and Asia-Pacific is accelerating adoption. Subscription-based service models provide additional growth momentum. With increasing interest in regenerative and personalized medicine, biobanks are expected to be the fastest-growing end user segment through 2032.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into direct sales, medical device/consumable distributors, contract cryobanking services, and e-commerce. The direct sales segment dominated the market with the largest revenue share in 2024, as manufacturers prioritize direct relationships with clinics, hospitals, and research centers. This approach ensures compliance with stringent handling requirements, reduces counterfeit risks, and allows tailored after-sales support. The ability to negotiate contracts for bulk purchases also drives its dominance. Strong networks with institutional buyers further solidify its leadership.
The contract cryobanking services segment is projected to record the fastest CAGR from 2025 to 2032, as more clinics and patients outsource adipose preservation to specialized providers. These services offer convenience, scalability, and cost-effectiveness by eliminating the need for in-house infrastructure. Providers also bundle additional services such as secure retrieval guarantees, value-added storage plans, and long-term preservation options. Growing networks of private cryobanks in Asia-Pacific and the Middle East are accelerating this shift. The outsourcing trend is set to fuel rapid adoption, making this the most dynamic growth channel over the forecast period.
Cryopreserved Fat Graft Viability Enhancement Solutions Market Regional Analysis
- North America dominated the cryopreserved fat graft viability enhancement solutions market with the largest revenue share of 39.2% in 2024, supported by high volumes of cosmetic surgery, advanced clinical infrastructure, and strong adoption of cryobanking services, with the U.S. leading in translational research and clinical implementation of adipose preservation protocols
- Clinics and hospitals in the region highly value the availability of optimized cryopreservation media, turnkey kits, and biobank services that ensure reliable fat graft viability and superior patient outcomes
- This widespread adoption is further supported by high healthcare expenditure, early regulatory approvals, and strong participation in clinical research programs, establishing North America as the leading hub for both clinical and commercial applications of cryopreserved fat graft solutions
U.S. Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The U.S. market captured the largest revenue share of 79% in 2024 within North America, fueled by the high volume of cosmetic fat grafting procedures and the rapid adoption of advanced cryopreservation technologies. Surgeons and patients increasingly prioritize graft survival rates, driving demand for optimized cryoprotectants and turnkey kits. The strong presence of biobanks and clinical research programs further accelerates innovation and accessibility. Moreover, regulatory clarity and FDA approvals for new formulations are reinforcing market confidence. The U.S. remains the key hub for both procedural adoption and product innovation in this field.
Europe Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The Europe market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent healthcare regulations and strong demand for reconstructive and aesthetic procedures. Growing awareness of long-term adipose preservation in both clinical and research applications is fostering adoption. European consumers and clinics are drawn to solutions that ensure higher graft viability, supported by compliance with EU medical device standards. The region is also experiencing growth in biobanking services, with fat tissue increasingly stored for regenerative and therapeutic purposes. Both hospitals and private clinics are integrating cryopreservation into standard practice.
U.K. Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The U.K. market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising demand for cosmetic procedures and regenerative medicine applications. Heightened concerns around procedural success and patient outcomes are encouraging surgeons to adopt advanced cryopreservation solutions. The U.K.’s well-established healthcare infrastructure and regulatory support provide a strong foundation for adoption. Furthermore, the rise of specialized fat banking services and collaborations with academic research centers is reinforcing the growth trajectory. Both private clinics and NHS-backed institutions are contributing to the expanding demand base.
Germany Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The Germany market is expected to expand at a considerable CAGR during the forecast period, supported by strong investments in life sciences and advanced preservation technologies. Germany’s focus on clinical excellence and innovation is fostering the use of novel cryoprotectant chemistries and controlled-rate freezing protocols. The country’s emphasis on sustainability and biocompatibility aligns with the development of next-generation preservation solutions. Hospitals and academic centers are playing a leading role in clinical validation, while private clinics are adopting turnkey kits for cosmetic procedures. This balance of innovation and application ensures consistent market growth.
Asia-Pacific Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The Asia-Pacific market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by increasing medical tourism, rising disposable incomes, and expanding cosmetic surgery volumes in countries such as China, Japan, and India. Government-backed healthcare modernization initiatives and digitalization programs are supporting wider adoption of cryopreservation systems. The region’s growing manufacturing capacity for consumables and storage devices is also improving affordability and accessibility. As aesthetic awareness rises among middle-class populations, demand for clinic-level and biobank-level fat graft storage is expanding rapidly across APAC.
Japan Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The Japan market is gaining momentum due to its advanced healthcare system, high demand for minimally invasive cosmetic procedures, and strong focus on regenerative medicine. Clinics and hospitals in Japan place significant emphasis on quality and reliability, driving adoption of vitrification and controlled-rate freezing methods. Integration of cryopreservation with other cell therapy and tissue engineering programs is also fueling growth. Moreover, Japan’s aging population is stimulating demand for reconstructive and aesthetic procedures that rely on high graft survival rates, further propelling the adoption of advanced viability enhancement solutions.
India Cryopreserved Fat Graft Viability Enhancement Solutions Market Insight
The India market accounted for the largest revenue share in Asia-Pacific in 2024, attributed to a rising middle-class population, expanding medical tourism sector, and strong adoption of cosmetic procedures. India is one of the fastest-growing markets for fat grafting, with patients increasingly seeking affordable and effective solutions. Domestic manufacturers are actively entering the cryopreservation solutions space, improving product availability. Furthermore, the government’s initiatives around healthcare digitalization and private sector investment in cosmetic surgery clinics are boosting adoption. Growing awareness of regenerative applications of adipose tissue is further strengthening India’s market outlook.
Cryopreserved Fat Graft Viability Enhancement Solutions Market Share
The Cryopreserved Fat Graft Viability Enhancement Solutions industry is primarily led by well-established companies, including:
- STEMCELL Technologies (Canada)
- Cryoport Systems, LLC (U.S.)
- Corning Incorporated (U.S.)
- Miltenyi Biotec (Germany)
- Cytiva (U.S.)
- Lonza (Switzerland)
- Tulip Medical (U.S.)
- Lipogems International S.p.A. (Italy)
- MTF Biologics (U.S.)
- AlloSource (U.S.)
- LifeLink Tissue Bank (U.S.)
- BioLife Solutions (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Terumo BCT, Inc. (U.S.)
- RegenLab SA (Switzerland)
- EmCyte Corporation (U.S.)
- American CryoStem Corporation (U.S.)
- Lipocube (U.S.)
What are the Recent Developments in Global Cryopreserved Fat Graft Viability Enhancement Solutions Market?
- In May 2025, researchers published a new cryopreservation solution based on metformin (called “TGM cryopreservation solution”) that preserved adipose tissue with lower apoptosis rates and higher retention in vivo, closely resembling fresh tissue in structure and viability. This offers a non-toxic, clinically promising alternative to traditional DMSO-based cryoprotectants
- In April 2025, BPB Medica™ introduced the LIPO-STEM DUO™ device, which avoids traditional centrifugation and instead uses continuous washing and microfragmentation for adipose tissue processing. While not strictly a “cryopreservation kit”, this device supports higher-quality tissue processing before freezing, which can improve graft viability
- In April 2025, American CryoStem Corporation announced the acquisition of BioLife Cell Bank’s adipose tissue and stem cell banking assets, strengthening its position as a provider of regenerative medicine cryostorage and expanding its physician network and recurring storage revenues
- In March 2024, LifeLink Tissue Bank announced a strategic collaboration with Britecyte®, Inc. to develop a platform technology for recovery, processing, and clinical application of adipose tissue. This joint effort introduced Liposana®, a regenerative medicine solution derived from adipose tissue, presented at the American College of Foot and Ankle Surgeons meeting
- In February 2024, a clinical-tech demonstration evaluated sequential fat grafting using fresh fat followed by cryopreserved stromal vascular fraction (SVF) gel for morphea treatment, illustrating practical clinical workflows that blend fresh and frozen adipose-derived components
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.