Global Cryptosporidiosis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
598.50 Billion
USD
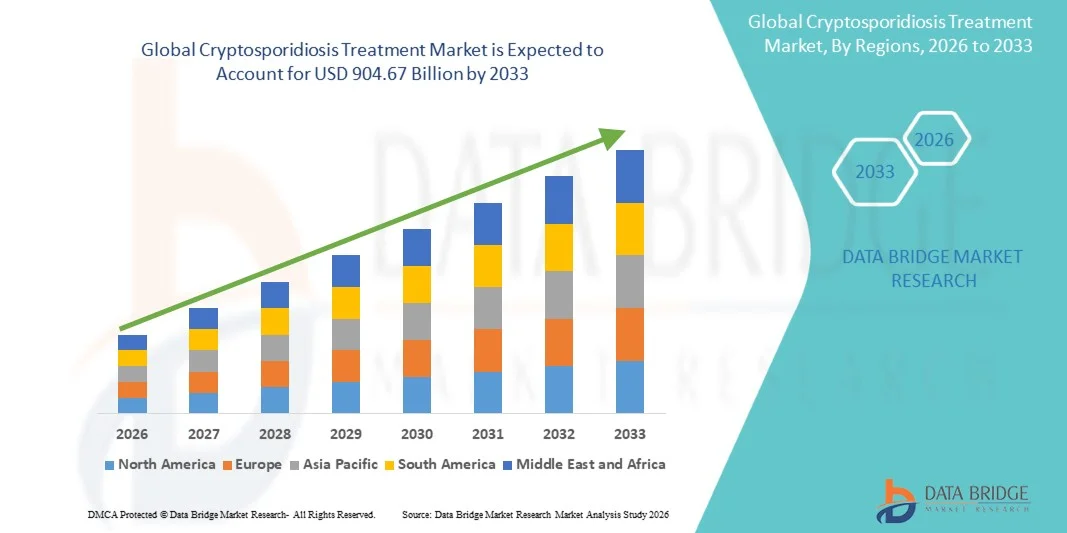
904.67 Billion
2025
2033
USD
598.50 Billion
USD
904.67 Billion
2025
2033
| 2026 –2033 | |
| USD 598.50 Billion | |
| USD 904.67 Billion | |
|
|
|
|
Cryptosporidiosis Treatment Market Size
- The global cryptosporidiosis treatment market size was valued at USD 598.5 Million in 2025 and is expected to reach USD 904.67 Million by 2033, at a CAGR of 5.30% during the forecast period
- The market growth is largely fueled by increasing awareness of waterborne and foodborne parasitic infections, improvements in diagnostic technologies, and the rising prevalence of immunocompromised populations such as HIV/AIDS patients, organ transplant recipients, and young children
- Furthermore, growing demand for effective therapeutic solutions and advancements in antiparasitic drugs and supportive care are driving the adoption of Cryptosporidiosis Treatment options, thereby significantly boosting the industry's growth
Cryptosporidiosis Treatment Market Analysis
- Cryptosporidiosis Treatment, encompassing antiparasitic drugs and supportive therapies, is increasingly vital in managing gastrointestinal infections in both immunocompromised and healthy populations due to its effectiveness, accessibility, and integration with hospital and outpatient care systems
- The escalating demand for cryptosporidiosis treatment is primarily fueled by rising prevalence of waterborne infections, growing awareness about early diagnosis, and increasing adoption of advanced therapeutic interventions, thereby driving substantial growth in the market
- North America dominated the cryptosporidiosis treatment market with the largest revenue share of approximately 35.8% in 2025, supported by advanced healthcare infrastructure, high disease awareness, widespread availability of specialized diagnostic and therapeutic solutions, and strong government-led initiatives for infectious disease management. The U.S. experienced substantial growth due to early detection, adoption of novel therapies, and expanded access to specialized healthcare centers
- Asia-Pacific is expected to be the fastest-growing region in the cryptosporidiosis treatment market during the forecast period, driven by increasing healthcare expenditure, rising prevalence of waterborne diseases, improved access to specialized care, and growing awareness about early diagnosis and treatment in countries such as India, China, and Japan
- The Ingestion of Contaminated Food or Water segment dominated the largest market revenue share of 50.4% in 2025, due to frequent waterborne and foodborne outbreaks
Report Scope and Cryptosporidiosis Treatment Market Segmentation
|
Attributes |
Cryptosporidiosis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cryptosporidiosis Treatment Market Trends
“Rising Focus on Innovative Therapies and Supportive Care”
- A notable trend in the global cryptosporidiosis treatment market is the increasing emphasis on developing innovative pharmacological and supportive care solutions to address severe and recurrent infections
- For instance, novel drug formulations and targeted therapies are being investigated to enhance parasite clearance rates and reduce treatment duration, offering improved patient outcomes
- Healthcare providers are increasingly adopting combination treatment approaches, integrating antiparasitic medication with hydration therapy and nutritional support
- Rising awareness about Cryptosporidiosis among clinicians and patients is driving demand for standardized treatment protocols and evidence-based interventions
- Advanced research initiatives are focusing on developing therapies suitable for immunocompromised individuals, such as HIV-positive patients and organ transplant recipients
- Pharmaceutical companies are collaborating with research institutions to accelerate the development of next-generation therapeutics and optimize treatment regimens
- The growing prevalence of waterborne and foodborne outbreaks in both developed and developing regions is further fueling research investment
- Emerging treatment modalities, including nanoparticle-based drug delivery systems, are gaining attention for enhanced efficacy and reduced side effects
Patient-centric care models are promoting early diagnosis and timely intervention, improving overall recovery rates - Government and non-government organizations are increasing funding for Cryptosporidiosis research and awareness programs
- Integration of clinical guidelines and digital health tools aids in consistent treatment administration and monitoring
- The trend toward personalized treatment strategies is expected to reshape therapeutic approaches in the coming years
Cryptosporidiosis Treatment Market Dynamics
Driver
“Growing Incidence and Need for Effective Treatment”
- The increasing global prevalence of cryptosporidiosis, particularly among children, immunocompromised patients, and populations in developing regions, is a key driver for market growth
- For instance, the World Health Organization has reported frequent outbreaks in areas with limited access to clean water and sanitation, prompting heightened focus on treatment and prevention
- Rising awareness about the health risks associated with Cryptosporidiosis is encouraging early diagnosis and medical intervention
- Healthcare providers are increasingly adopting evidence-based treatment protocols to reduce morbidity and hospitalization rates. The demand for efficient antiparasitic medications is amplified by recurrent infections and drug resistance concerns
- Pharmaceutical companies are expanding their product portfolios to include both first-line and supportive therapies. Improved diagnostic capabilities, including advanced laboratory testing, support timely treatment initiation and monitoring
- Non-governmental organizations and public health campaigns are promoting preventive measures, indirectly supporting treatment adoption
- The burden of diarrheal diseases in pediatric populations is creating an urgent need for targeted Cryptosporidiosis therapies
- Emerging markets are witnessing increased access to healthcare infrastructure, fueling adoption of standard treatment regimens
- Rising healthcare expenditure in key regions supports development and distribution of effective therapies
- Investment in training healthcare professionals for proper management of Cryptosporidiosis is enhancing treatment penetration
Restraint/Challenge
“Concerns Regarding High Treatment Costs and Limited Access”
- The relatively high cost of advanced Cryptosporidiosis therapies compared to conventional supportive care can limit access, particularly in low-income regions
- For instance, patients in rural or resource-limited areas often face challenges in obtaining prescription medications or completing full treatment courses
- Inconsistent availability of key drugs in hospitals and pharmacies can hinder effective treatment administration
- Lack of standardized treatment protocols in some regions may result in suboptimal patient outcomes
- Concerns regarding potential side effects of antiparasitic drugs contribute to cautious prescription practices
- Educational gaps among healthcare providers regarding best practices for Cryptosporidiosis management affect market adoption
- Regulatory hurdles and slow approval of new therapeutics can delay market entry of innovative treatments
- Insufficient healthcare infrastructure in developing regions impacts timely diagnosis and treatment
- Variability in patient adherence to prescribed treatment regimens can limit therapy effectiveness
- High treatment costs may prevent long-term therapy access for recurrent or chronic cases
- Addressing these challenges requires strategic partnerships, government support, and awareness programs to improve access
- Developing affordable therapies and expanding distribution networks are critical for sustained market growth
Cryptosporidiosis Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, symptoms, mode of transmission, dosage, route of administration, end-users, and distribution channel.
• By Treatment
On the basis of treatment, the Cryptosporidiosis Treatment market is segmented into Anti-parasitic Drugs, Anti-motility Agents, Antiretroviral Therapies, Fluid Replacement, and Others. The Anti-parasitic Drugs segment dominated the largest market revenue share of 45.6% in 2025, owing to its high efficacy in clearing Cryptosporidium infections and widespread clinical adoption. Physicians prefer anti-parasitic therapies for both pediatric and immunocompromised patients due to their proven effectiveness. The segment benefits from ongoing research to improve drug formulations and delivery methods, enhancing patient compliance. Availability across hospitals, clinics, and pharmacies contributes to steady market demand. Increasing awareness of Cryptosporidiosis and its complications further boosts adoption. Government and non-government health initiatives promoting standard treatment protocols also support growth. The segment is well-established in both developed and developing regions. Rising prevalence of chronic infections and recurring cases drives continuous need. Clinical guidelines frequently recommend anti-parasitic drugs as first-line therapy. Education and training of healthcare professionals further reinforce their widespread use. Overall, this segment remains central to treatment strategies, representing a stable revenue contributor.
The Fluid Replacement segment is expected to witness the fastest CAGR of 22.1% from 2026 to 2033, driven by the critical need to manage dehydration caused by acute diarrhea in Cryptosporidiosis patients. Hospitals increasingly emphasize supportive care alongside drug therapy. Growth is fueled by rising awareness of electrolyte imbalance risks. Pediatric and elderly populations benefit particularly from this therapy. Expansion of hospital infrastructure in emerging regions boosts accessibility. Increasing incidence of severe cases in immunocompromised individuals further supports demand. Clinical protocols are increasingly integrating fluid replacement for comprehensive care. The development of ready-to-use oral rehydration solutions accelerates adoption. NGOs and public health programs promote fluid therapy for at-risk populations. Rising focus on outpatient care enhances market penetration. Improved formulations for faster absorption are contributing to segment growth. Regulatory approvals of safer intravenous solutions further support expansion.
• By Diagnosis
On the basis of diagnosis, the market is segmented into Acid-staining Test, Stool Culture, and Others. The Stool Culture segment held the largest revenue share of 41.3% in 2025, owing to its accuracy in detecting Cryptosporidium oocysts. Widely available in clinical laboratories, it is preferred for confirming infections in both acute and chronic cases. The method supports evidence-based treatment decisions. Hospitals and diagnostic centers in urban areas drive revenue growth. Standardization of testing protocols across regions enhances reliability. Increasing laboratory capacity in developing nations also boosts adoption. Early detection in pediatric and immunocompromised patients is a key factor. Clinical guidelines prioritize stool culture for high-risk groups. Integration with hospital management systems streamlines reporting. Rising prevalence of gastroenteritis in children fuels testing demand. Training programs for laboratory personnel improve testing efficiency. Overall, stool culture remains a cornerstone in Cryptosporidiosis diagnostics.
The Acid-staining Test segment is expected to witness the fastest CAGR of 19.8% from 2026 to 2033, due to its rapid and cost-effective detection capability, particularly in resource-limited regions. Growing demand for point-of-care diagnostics accelerates adoption. It is favored in field hospitals and rural clinics. Easy implementation by laboratory technicians supports widespread use. Rising awareness campaigns emphasize early detection. Technological improvements are enhancing sensitivity and specificity. Integration with mobile diagnostic tools is emerging. Frequent outbreaks in developing regions drive repeated testing. Non-governmental health programs encourage adoption in community settings. Cost advantages make it accessible for low-income patients. Enhanced supply chains improve availability. Faster turnaround times strengthen its role in acute management.
• By Symptoms
On the basis of symptoms, the market is segmented into Fever, Watery Diarrhea, Lack of Appetite, Stomach Cramps, Weight Loss, Nausea, Vomiting, Dehydration, and Others. The Watery Diarrhea segment dominated the largest revenue share of 47.2% in 2025, as it is the most common presenting symptom driving clinical consultation. High prevalence in pediatric and immunocompromised populations increases treatment demand. Public health campaigns highlight the importance of addressing diarrhea promptly. Hospital admissions for acute diarrhea contribute to segment growth. Clinical management protocols prioritize hydration and monitoring. Awareness among caregivers further supports early intervention. Symptom-based diagnosis often guides treatment choice. Epidemiological studies reinforce the burden of diarrheal cases. Healthcare spending for symptomatic relief sustains market revenue. Nutritional support programs complement therapy. Rising prevalence in both urban and rural regions drives consistent demand.
The Dehydration segment is expected to witness the fastest CAGR of 21.5% from 2026 to 2033, due to critical care needs in severe Cryptosporidiosis cases. Outpatient and hospital-based fluid replacement therapy fuels growth. Pediatric populations are especially at risk. Emergency care adoption increases segment penetration. Development of oral and IV rehydration solutions accelerates market growth. Awareness of electrolyte imbalance risks drives early treatment. NGO programs support rural adoption. Rising focus on holistic patient management enhances uptake. Technological improvements in rehydration formulations expand options. Training healthcare providers strengthens adoption. High incidence of chronic diarrheal cases drives repeat use. Government initiatives promote standardized care protocols.
• By Mode of Transmission
On the basis of mode of transmission, the market is segmented into Person-to-Person, Animal-to-Person, and Ingestion of Contaminated Food or Water. The Ingestion of Contaminated Food or Water segment dominated the largest market revenue share of 50.4% in 2025, due to frequent waterborne and foodborne outbreaks. Contaminated water supplies in developing regions drive recurrent infections. Public awareness campaigns on safe water practices support early treatment demand. Healthcare providers prioritize patients exposed to contaminated sources. Outbreak management strategies reinforce segment growth. Epidemiological surveillance identifies high-risk populations. Urbanization and industrialization contribute to exposure risk. Hospital treatment protocols emphasize rapid intervention. Laboratory testing for exposure assessment strengthens clinical decision-making. Government regulations on water safety indirectly boost treatment adoption. Community health programs further increase awareness. Overall, ingestion-related infections remain the leading cause of therapy demand.
The Person-to-Person segment is expected to witness the fastest CAGR of 20.2% from 2026 to 2033, driven by increasing household and daycare outbreaks. Pediatric care facilities contribute to segment expansion. Growing institutional awareness of hygiene practices supports adoption. Recurrent infections in caregivers and family members enhance treatment demand. Surveillance programs track transmission trends. Early detection and preventive care strengthen segment penetration. Schools and clinics emphasize patient education. NGO programs targeting hygiene awareness accelerate growth. Immunocompromised populations are especially prioritized. Effective treatment reduces secondary infection risks. Rapid point-of-care diagnostics improve management. Hospital infection control protocols further drive adoption.
• By Dosage
On the basis of dosage, the market is segmented into Tablet, Injection, and Others. The Tablet segment held the largest market revenue share of 46.7% in 2025, due to convenience, ease of administration, and patient compliance. Widespread prescription of oral anti-parasitic drugs supports dominance. Pediatric-friendly formulations enhance adoption. Hospital and outpatient treatment protocols frequently utilize tablets. Cost-effectiveness favors usage in emerging regions. Standardized dosing regimens increase clinician preference. Availability through hospital and retail pharmacies sustains revenue. Tablets are suitable for long-term therapy in chronic cases. Patient adherence programs improve treatment completion. Government subsidies in some regions further drive uptake. Clinical guidelines recommend tablets as first-line therapy. Tablets offer minimal invasiveness, enhancing patient comfort.
The Injection segment is expected to witness the fastest CAGR of 19.9% from 2026 to 2033, driven by critical care needs in severe or hospitalized cases. Intravenous administration ensures rapid therapeutic action. Adoption in hospital settings is increasing. Pediatric and immunocompromised patients benefit from injections. Emergence of combination injectable therapies boosts growth. Improved safety profiles enhance clinician confidence. Government and NGO programs support injectable therapy availability. Emergency care adoption accelerates usage. Intravenous therapy complements supportive care. Advanced formulations increase effectiveness. Repeat therapy in severe cases drives market expansion. Expansion of hospital infrastructure further fuels growth.
• By Route of Administration
On the basis of route of administration, the market is segmented into Oral, Intravenous, and Others. The Oral segment dominated the largest market revenue share of 48.1% in 2025, owing to convenience and wide availability. High patient compliance and outpatient suitability boost adoption. Standard treatment protocols favor oral administration for most Cryptosporidiosis cases. Hospitals and clinics widely dispense oral medications. Pediatric-friendly formulations increase penetration. Cost-effectiveness supports market growth in emerging regions. Oral therapy integrates easily with supportive care regimens. NGOs and government programs distribute oral therapy in endemic areas. Patient adherence programs further support uptake. Availability in retail and hospital pharmacies sustains dominance. Early-stage infections are often treated orally. Oral formulations remain the preferred mode of administration globally.
The Intravenous segment is expected to witness the fastest CAGR of 20.3% from 2026 to 2033, driven by severe and hospitalized cases requiring rapid intervention. Adoption in emergency and inpatient care increases. Pediatric and immunocompromised patients benefit most. Technological advances in IV formulations enhance efficacy. Clinical protocols emphasize IV therapy for severe dehydration. Hospital infrastructure expansion supports growth. Emergency treatment programs strengthen market penetration. Repeat therapy for acute cases boosts usage. NGO and government initiatives improve IV therapy access. Rapid action of IV therapy improves patient outcomes. Combination with supportive care accelerates adoption. Regulatory approvals of new IV formulations further fuel growth.
• By End-Users
On the basis of end-users, the market is segmented into Clinic, Hospital, and Others. The Hospital segment held the largest market revenue share of 52.3% in 2025, driven by high patient inflow and availability of comprehensive treatment facilities. Hospitals manage both outpatient and inpatient cases efficiently. Adoption of standardized treatment protocols enhances effectiveness. Hospital pharmacies ensure drug availability. Pediatric and immunocompromised care requires hospital-based monitoring. Advanced diagnostic facilities support treatment decisions. Trained medical staff increase quality of care. Hospitals are primary sites for severe and recurrent cases. Insurance coverage in hospitals boosts accessibility. NGO collaborations support hospital treatment programs. Hospitals lead clinical research and adoption of innovative therapies. Patient preference for hospital care drives revenue dominance.
The Clinic segment is expected to witness the fastest CAGR of 21.0% from 2026 to 2033, due to growing outpatient care facilities in urban and semi-urban regions. Clinics provide early-stage management of mild infections. Easy accessibility and reduced cost favor clinics. Pediatric and community clinics expand treatment reach. Adoption of standardized outpatient protocols accelerates growth. NGO health programs support clinic-based care. Increasing patient awareness boosts clinic visits. Rapid diagnostics in clinics aid timely treatment. Clinics serve as primary points for preventive care. Private clinics contribute to market expansion. Integration with retail pharmacies supports drug access. Growing focus on community-level treatment drives adoption.
• By Distribution Channel
On the basis of distribution channel, the market is segmented into Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy. The Hospital Pharmacy segment dominated the largest market revenue share of 49.8% in 2025, due to direct access to prescribed therapies and integration with hospital care. Hospitals ensure uninterrupted supply for inpatient and outpatient cases. Collaboration with healthcare providers enhances adoption. Availability of advanced therapies in hospital pharmacies boosts revenue. Pediatric and immunocompromised patient access drives demand. Standardized protocols reinforce pharmacy usage. Hospital pharmacy networks in emerging regions support penetration. Insurance coverage facilitates patient access. Hospitals provide counseling and adherence support. Clinical research integration strengthens market relevance. Government programs channel drugs through hospital pharmacies. Hospitals remain central to therapy distribution globally.
The Online Pharmacy segment is expected to witness the fastest CAGR of 23.2% from 2026 to 2033, driven by growing e-commerce adoption, convenience, and increasing telemedicine integration. Online platforms provide access in remote areas. Home delivery improves patient adherence. Growing acceptance of digital health services accelerates growth. Retail and clinic tie-ups enhance availability. E-prescriptions simplify purchase and tracking. Cost comparison tools support informed decisions. Telehealth consultations drive online demand. Awareness campaigns promote online access. Emerging regions adopt digital platforms for wider reach. Private partnerships strengthen online distribution. Seasonal demand for Cryptosporidiosis treatment boosts online sales. Regulatory approvals ensure safe online dispensing.
Cryptosporidiosis Treatment Market Regional Analysis
- North America dominated the cryptosporidiosis treatment market with the largest revenue share of approximately 35.8% in 2025, supported by advanced healthcare infrastructure, high disease awareness, widespread availability of specialized diagnostic tools, and strong government initiatives aimed at monitoring and controlling waterborne infections
- The region also benefits from robust research activities, improved access to anti-parasitic therapies, and well-established surveillance systems that enable early diagnosis and timely intervention
- Increased investment in infectious disease management, coupled with rising cases linked to contaminated recreational water, further strengthens North America’s leadership in this market
U.S. Cryptosporidiosis Treatment Market Insight
The U.S. cryptosporidiosis treatment market captured around of the North American Cryptosporidiosis Treatment market in 2025, driven by early adoption of advanced diagnostic technologies, high healthcare spending, and a strong clinical focus on waterborne disease management. The country continues to experience rising demand for accurate stool testing, improved anti-parasitic formulations, and enhanced treatment accessibility across hospitals, clinics, and public health laboratories. Ongoing CDC-supported surveillance programs and investments in molecular diagnostic methods significantly contribute to U.S. market expansion.
Europe Cryptosporidiosis Treatment Market Insight
The Europe cryptosporidiosis treatment market is projected to expand at a substantial CAGR during the forecast period, driven by rising awareness of parasitic infections, improvements in water-quality monitoring, and enhanced diagnostic capabilities throughout the region. Increased urbanization, strict health regulations, and the growing focus on preventing outbreaks in hospitals, childcare centers, and aged-care facilities are supporting market growth. Europe is also seeing rising adoption of molecular and rapid diagnostic tests across both public and private healthcare sectors.
U.K. Cryptosporidiosis Treatment Market Insight
The U.K. cryptosporidiosis treatment market is expected to grow at a noteworthy CAGR, driven by increased surveillance of waterborne outbreaks and expanding use of advanced diagnostic tools in healthcare settings. Rising cases associated with contaminated drinking and recreational water, along with strong government health initiatives, are contributing to the adoption of improved treatment protocols. The U.K.’s emphasis on early diagnosis and robust public-health reporting systems further supports market expansion.
Germany Cryptosporidiosis Treatment Market Insight
Germany’s cryptosporidiosis treatment market is anticipated to expand at a considerable CAGR, supported by strong investment in public health, advanced laboratory infrastructure, and increasing attention to digital health surveillance. The country’s focus on precision diagnostics, sustainable healthcare practices, and stringent hygiene regulations promotes increasing diagnostic rates and supports the demand for effective therapeutic solutions.
Asia-Pacific Cryptosporidiosis Treatment Market Insight
The Asia-Pacific cryptosporidiosis treatment market is projected to grow at the fastest CAGR from 2026 to 2033, driven by rising prevalence of waterborne diseases, increasing healthcare expenditure, and improved access to diagnostic and treatment services across major countries such as China, India, and Japan. Government-led initiatives promoting disease awareness, water sanitation programs, and the expansion of healthcare infrastructure are accelerating market growth. APAC’s population growth and higher incidence of diarrheal infections also contribute significantly to demand.
Japan Cryptosporidiosis Treatment Market Insight
Japan’s cryptosporidiosis treatment market is witnessing steady momentum due to strong public-health awareness, rapid urbanization, and rapid adoption of advanced medical technologies. The country’s aging population—more vulnerable to dehydration and gastrointestinal infections—drives demand for precise diagnosis and effective treatments. Integration of high-sensitivity diagnostic systems within hospitals and clinics further supports market expansion.
China Cryptosporidiosis Treatment Market Insight
China cryptosporidiosis treatment market accounted for the largest revenue share within Asia-Pacific in 2025, fueled by increasing urbanization, a rapidly expanding middle class, and growing government initiatives to improve water-quality monitoring. China's large population base and rising incidence of diarrheal diseases are boosting the need for efficient diagnostic tools and accessible therapies. The country's strong domestic manufacturing capabilities and expanding healthcare reforms further support growth in the Cryptosporidiosis Treatment market.
Cryptosporidiosis Treatment Market Share
The Cryptosporidiosis Treatment industry is primarily led by well-established companies, including:
- Gilead Sciences (U.S.)
- Johnson & Johnson (U.S.)
- GlaxoSmithKline (U.K.)
- Pfizer (U.S.)
- Roche (Switzerland)
- Novartis (Switzerland)
- Astellas Pharma (Japan)
- Bayer (Germany)
- Cipla (India)
- Sanofi (France)
- Merck & Co. (U.S.)
- Takeda Pharmaceutical (Japan)
- AbbVie (U.S.)
- Sun Pharmaceutical (India)
- Shionogi & Co. (Japan)
- Fresenius Kabi (Germany)
- Mylan (U.S.)
- Teva Pharmaceuticals (Israel)
- Shanghai Pharmaceuticals (China)
- Servier (France)
Latest Developments in Global Cryptosporidiosis Treatment Market
- In July 2021, researchers published a study showing that a new prodrug, Aminoxanide (RM‑5061), had strong anti‑Cryptosporidium activity in both in vitro assays and immunosuppressed animal models. The 5‑day intramuscular treatment in gerbils reduced oocyst excretion by ~72.5%, similar to oral treatment with the standard drug, offering a potential injectable alternative for severe or immunosuppressed cases
- In November 2024, scientists at University of Dundee announced discovery of two novel chemical compounds that showed promising efficacy in animal models of cryptosporidiosis, giving hope for the first new class of treatments in decades. These compounds were optimized to penetrate the gut (the main infection site) rather than focus solely on solubility — a key innovation given the parasite’s gut localization
- In October 2024, a study published in Science Translational Medicine reported two new compounds (DDD489 and DDD508) that achieved over 99.8% parasite reduction in a mouse model of cryptosporidiosis, with no relapse or recrudescence; treated calves also showed significantly reduced diarrheal severity. These compounds have been selected for preclinical safety studies, indicating a potential breakthrough in cryptosporidiosis therapy
- In October 2025, a research group led by a professor at University of Houston received a ~USD4 million grant from the National Institute of Allergy and Infectious Diseases (NIAID) to advance enzyme‑targeting drug discovery against cryptosporidiosis. The target: CDPK1 (Calcium‑dependent protein kinase 1), a parasite enzyme shown to be essential for Cryptosporidium survival — marking a major step toward developing novel therapeutics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.