Global Dendritic Cell Therapy Vaccine Market
Market Size in USD Billion
CAGR :
% 
 USD
9.73 Billion
USD
14.65 Billion
2024
2032
USD
9.73 Billion
USD
14.65 Billion
2024
2032
| 2025 –2032 | |
| USD 9.73 Billion | |
| USD 14.65 Billion | |
|
|
|
|
Dendritic Cell Therapy Vaccine Market Size
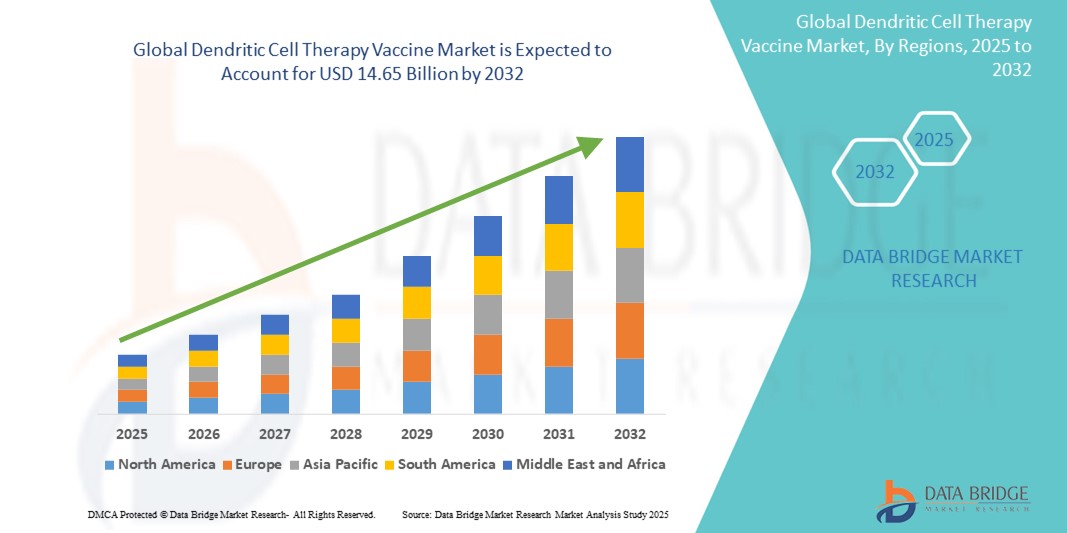
- The global dendritic cell therapy vaccine market was valued at USD 9.73 billion in 2024 and is expected to reach USD 14.65 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 5.25%, primarily driven by increasing cancer prevalence and the growing demand for personalized immunotherapies.
- This growth is driven by factors such as the advancements in cancer immunotherapy, rising adoption of personalized medicine, and increasing investments in research and development of dendritic cell-based vaccines
Dendritic Cell Therapy Vaccine Market Analysis
- Dendritic cell therapy vaccines are innovative immunotherapeutic treatments designed to enhance the immune system's ability to target and destroy cancer cells. These vaccines use a patient’s dendritic cells to stimulate an immune response, primarily targeting cancers such as melanoma, prostate cancer, and glioblastoma
- The demand for dendritic cell therapy vaccines is driven by the rising global cancer prevalence, the growing focus on personalized medicine, and the advancements in immunotherapy technologies. As these therapies are highly tailored to individual patients, they are gaining traction in cancer treatment
- North America is a key player in the dendritic cell therapy vaccine market, with its advanced healthcare infrastructure, substantial research funding, and high adoption of cutting-edge immunotherapies
- For instance, the U.S. is home to numerous clinical trials and research studies focused on dendritic cell vaccines, further driving the growth of the market
- Globally, dendritic cell therapy vaccines are gaining recognition as a critical component in personalized cancer treatment strategies, offering improved outcomes and survival rates for patients through more targeted immune responses
Report Scope and Dendritic Cell Therapy Vaccine Market Segmentation
|
Attributes |
Dendritic Cell Therapy Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Dendritic Cell Therapy Vaccine Market Trends
“Growing Focus on Combination Therapies and Personalized Medicine”
- A prominent trend in the global dendritic cell therapy vaccine market is the increasing focus on combination therapies and personalized medicine
- Dendritic cell vaccines are being increasingly combined with other treatment modalities, such as checkpoint inhibitors or chemotherapy, to enhance their efficacy and boost the immune response against cancer
- For instance, combining dendritic cell vaccines with checkpoint inhibitors has shown promise in enhancing the body’s ability to attack tumor cells, offering a more comprehensive treatment strategy
- Personalized medicine is at the core of dendritic cell vaccine development, as these vaccines are tailored to the individual patient's immune system and cancer profile, making treatments more targeted and effective
- This trend is improving treatment outcomes, offering hope for patients with cancers that are difficult to treat using conventional methods, and driving the demand for innovative immunotherapies in the market
Dendritic Cell Therapy Vaccine Market Dynamics
Driver
“Rising Cancer Prevalence and Shift Towards Immunotherapy”
- The increasing prevalence of cancer globally is significantly driving the demand for dendritic cell therapy vaccines. As cancer rates rise, there is a greater need for innovative and effective treatment options
- Cancer is one of the leading causes of death worldwide, and traditional treatment methods such as chemotherapy and radiation often come with limitations
- As a result, more patients and healthcare providers are seeking alternative treatments such as dendritic cell vaccines, which are more personalized and can boost the immune system’s natural ability to fight tumors
- The dendritic cell therapy vaccines are particularly promising for cancers that are difficult to treat with conventional therapies, such as melanoma, prostate cancer, and glioblastoma. The vaccines stimulate the immune system, targeting specific cancer cells while minimizing damage to healthy tissue
- In addition, the global shift towards immunotherapy as a primary treatment modality is accelerating the adoption of dendritic cell vaccines. Immunotherapies are gaining recognition for their ability to provide long-term, durable responses in cancer patients
- As the global population ages, the incidence of cancer continues to rise, making the demand for dendritic cell therapy vaccines even more significant. This demographic shift is a key factor fueling the growth of the market
For instance,
- According to the American Cancer Society, over 1.9 million new cancer cases were expected to be diagnosed in the U.S. in 2022, highlighting the increasing demand for advanced immunotherapies such as dendritic cell vaccines
- A report from the World Health Organization (WHO) states that cancer is the second leading cause of death globally, emphasizing the growing need for innovative treatment options, including dendritic cell-based therapies
- The rising cancer prevalence and increasing reliance on immunotherapy are key drivers for the global dendritic cell therapy vaccine market, creating significant demand for this advanced therapeutic approach
Opportunity
“Leveraging Combination with Other Immunotherapies for Enhanced Efficacy”
- One significant opportunity in the global dendritic cell therapy vaccine market is the growing potential to combine these vaccines with other immunotherapies, such as checkpoint inhibitors, monoclonal antibodies, and CAR-T cell therapies, to enhance overall treatment efficacy
- By combining dendritic cell vaccines with these therapies, it is possible to increase the activation and effectiveness of the immune system in attacking cancer cells, offering a more comprehensive and robust therapeutic approach
- In addition, the synergy between dendritic cell vaccines and other immunotherapies can help overcome resistance mechanisms that tumors develop against single therapies, improving patient outcomes and extending survival rates
For instance,
- In September 2023, a study published in Nature Reviews Cancer showed that combining dendritic cell vaccines with PD-1 inhibitors resulted in improved survival rates in patients with advanced melanoma, highlighting the power of combination therapies in cancer treatment
- In 2024 article in The Lancet Oncology revealed that combining dendritic cell vaccines with CAR-T therapies has the potential to target both the tumor microenvironment and circulating cancer cells, significantly improving treatment responses in patients with hematologic cancer
- As research progresses, the combination of dendritic cell vaccines with other immunotherapies is expected to become a standard approach in cancer treatment, providing a significant growth opportunity in the market
Restraint/Challenge
“High Development and Production Costs Hindering Widespread Adoption”
- The high costs associated with the development and production of dendritic cell therapy vaccines present a significant challenge for the market, limiting accessibility and affordability, especially in low-income regions
- The complex and personalized nature of dendritic cell vaccines requires advanced infrastructure, skilled personnel, and extensive research, all of which contribute to the high production costs. In addition, the manufacturing process is highly specialized and time-consuming, further elevating costs
- These financial barriers can prevent smaller clinics and hospitals from offering dendritic cell therapy vaccines to their patients, especially in regions with limited healthcare resources, thereby restricting access to this promising treatment
For instance,
- According to a 2023 study published in Vaccine by the World Health Organization, the cost of production and the lack of reimbursement policies in some countries are major obstacles to making dendritic cell vaccines widely accessible
- Consequently, these cost-related challenges can slow down the adoption of dendritic cell therapy vaccines, limiting their potential market growth and accessibility for a broader patient population. The need for cost-effective production methods and better reimbursement systems remains a key challenge for the market's expansion
Dendritic Cell Therapy Vaccine Market Scope
The market is segmented on the basis of product and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
By End User |
|
Dendritic Cell Therapy Vaccine Market Regional Analysis
“North America is the Dominant Region in the Dendritic Cell Therapy Vaccine Market”
- North America holds the largest share of the global dendritic cell therapy vaccine market, driven by its advanced healthcare infrastructure, strong presence of biotechnology and pharmaceutical companies, and high adoption of innovative medical therapies
- U.S. is the key player in this market, supported by substantial investments in cancer research, numerous clinical trials, and a favorable regulatory environment for new treatments. The increasing number of cancer cases and the growing demand for personalized immunotherapies further propel the market growth
- In addition, well-established reimbursement systems and a high level of healthcare awareness contribute to the growing adoption of dendritic cell vaccines in the region
- The region also benefits from a strong presence of key market players focusing on the development and commercialization of dendritic cell vaccines, bolstering research and development activities in the field
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to witness the highest growth rate in the dendritic cell therapy vaccine market, driven by rapid advancements in healthcare infrastructure, increasing cancer prevalence, and expanding access to innovative treatments
- Countries such as China, India, and Japan are emerging as key markets due to their large patient populations, rising cancer rates, and increasing focus on personalized medicine
- Japan continues to lead in cancer research and the adoption of cutting-edge medical technologies, making it a crucial market for dendritic cell therapy vaccines. The country is also investing heavily in developing immunotherapies to address various types of cancer
- China and India, with their expanding healthcare systems and increasing government support for cancer research, are witnessing significant growth in the demand for advanced cancer treatments, including dendritic cell vaccines. The rising awareness about cancer prevention and treatment in these countries is further driving market expansion
Dendritic Cell Therapy Vaccine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Argos Therapeutics (U.S)
- Batavia Biosciences B.V. (Netherlands)
- Bellicum Pharmaceuticals Inc. (U.S)
- JW CreaGene (South Korea)
- Mendus (Netherlands)
- ELIOS THERAPEUTICS, LLC (U.S)
- EOM Pharmaceuticals, Inc. (U.S)
- Kiromic BioPharma, Inc. (U.S)
- Medigene AG (Germany)
- Merck & Co., Inc. (U.S)
- Northwest Biotherapeutics, Inc. (U.S)
- GSK plc. (U.S)
- tella Inc. (China)
- VAXIL BIO THERAPEUTICS (Canada)
- CureVac SE (Germany)
- OncoOne (U.S)
- Diakonos Oncology Corporation (U.S.)
Latest Developments in Global Dendritic Cell Therapy Vaccine Market
- In March 2025, researchers at Moffitt Cancer Center developed a dendritic cell vaccine targeting HER2-positive, estrogen receptor-negative breast cancer. Early studies suggest it can enhance chemotherapy effectiveness in this aggressive cancer subtype
- In January 2025, DOC1021, an autologous dendritic cell vaccine, demonstrated a 93% 12-month survival rate in glioblastoma patients. The vaccine has received FDA Fast Track and Orphan Drug designations, with a Phase 2 trial expected to begin in Q1 2025
- In December 2024, the FDA granted Fast Track designation to Diakonos Oncology's dendritic cell vaccine for pancreatic ductal adenocarcinoma. This vaccine aims to activate robust immune responses without modifying the patient's cells
- In September 2024, a first-in-human clinical trial commenced for WDVAX, a dendritic cell vaccine incorporating autologous tumor cell lysate, to evaluate its safety and immunogenicity in patients with advanced cancers
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.