Global Dermatology Small Molecule Api Market
Market Size in USD Billion
CAGR :
% 
 USD
3.64 Billion
USD
6.26 Billion
2024
2032
USD
3.64 Billion
USD
6.26 Billion
2024
2032
| 2025 –2032 | |
| USD 3.64 Billion | |
| USD 6.26 Billion | |
|
|
|
Dermatology Small Molecule API Market Analysis
The dermatology small molecule API market is experiencing steady growth driven by the increasing incidence of skin disorders such as acne, psoriasis, eczema, and atopic dermatitis. The rising awareness about skin health, coupled with the demand for more affordable treatment options, is propelling the market forward. Small molecule drugs, particularly those targeting inflammatory pathways, are being increasingly adopted due to their cost-effectiveness and ease of use compared to biologics.
A key driver for the market is the growing preference for topical small molecule therapies over systemic treatments, particularly for conditions such as acne and psoriasis. Topical drugs such as tretinoin, a retinoid, and topical corticosteroids continue to dominate the treatment of mild to moderate skin conditions due to their efficacy and relatively lower cost. Additionally, the development of novel small molecules targeting specific molecular pathways such as JAK inhibitors for atopic dermatitis and psoriasis is creating new opportunities for growth. The continued shift towards personalized medicine, where treatments are tailored to an individual's specific skin condition and genetic profile, is further boosting demand for dermatology small molecule APIs, offering promising market prospects.
Dermatology Small Molecule API Market Size
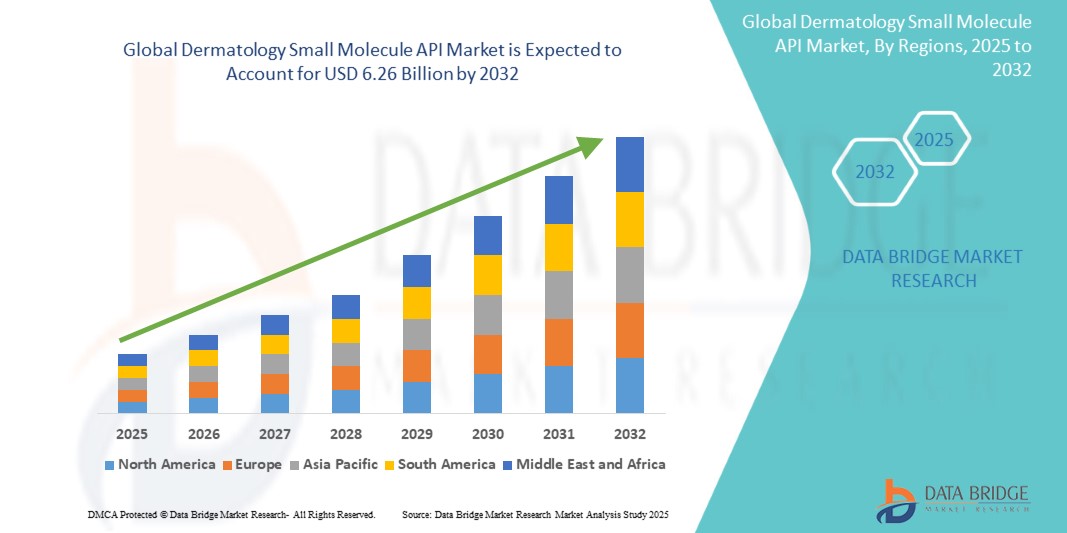
The global dermatology small molecule API market size was valued at USD 3.64 billion in 2024 and is projected to reach USD 6.26 billion by 2032, with a CAGR of 7.00% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Dermatology Small Molecule API Market Trends
“Rising Prevalence of Chronic Skin Disorders”
The main reason for the growth of the dermatology small molecule API market is the rising prevalence of chronic skin disorders such as acne, psoriasis, eczema, and atopic dermatitis, coupled with increasing patient demand for more effective and affordable treatments. As these conditions become more common worldwide, there is a growing need for both topical and systemic therapies that can manage symptoms and improve quality of life.
Small molecule drugs are gaining traction due to their cost-effectiveness, accessibility, and proven efficacy, particularly in the treatment of mild to moderate skin conditions. For instance, topical retinoids such as tretinoin and corticosteroids remain first-line treatments for acne and psoriasis, offering patients targeted relief at a lower cost compared to biologics. Additionally, the development of newer, more advanced small molecule therapies, such as JAK inhibitors for inflammatory skin diseases, is expanding the treatment options available, fueling further market growth. The shift towards personalized dermatology treatments is also driving demand, with tailored therapies increasingly sought after.
Report Scope and Dermatology Small Molecule API Market Segmentation
|
Attributes |
Dermatology Small Molecule API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Abbott (U.S.), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Arcutis Biotherapeutics, Inc. (U.S.), BIOCAD HK Ltd (China), Bristol-Myers Squibb Company (U.S.), Can-Fite (Israel), CELLTRION INC. (Republic of Korea), Coherus BioSciences (U.S.), DermBiont, Inc. (U.S.), Galectin Therapeutics, Inc. (U.S.), Galderma (Switzerland), ILTOO Pharma (France), GSK plc. (U.K.), Johnson & Johnson Services, Inc. (U.S.), LEO Pharma A/S (Denmark), Pfizer Inc. (U.S.), Roivant Sciences Ltd. (U.K.), Sun Pharmaceutical Industries Ltd. (India), and Viatris Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Dermatology Small Molecule API Market Definition
Dermatology small molecule API refers to active pharmaceutical ingredients (APIs) that are small molecules specifically developed for the treatment of dermatological conditions. These molecules are typically low molecular weight compounds that interact with specific biological targets in the body to treat skin diseases or conditions.
Dermatology Small Molecule API Market Dynamics
Drivers
- Advancements in Drug Delivery Systems
Technological advancements in drug delivery systems, including sustained-release formulations, transdermal systems, and nano-drug delivery technologies, have significantly enhanced the effectiveness and bioavailability of small molecule drugs. These innovations not only improve patient compliance but also optimize treatment outcomes, particularly for chronic dermatological conditions. For instance, Tretinoin (Retin-A), a widely used treatment for acne and aging signs, is now available in sustained-release formulations that boost its therapeutic efficacy while minimizing irritation. By enabling a controlled release of the drug, sustained-release technologies provide consistent therapeutic effects over time, enhancing long-term patient outcomes. These advancements in drug delivery systems serve as a major driver for the growth of the Dermatology Small Molecule API Market.
- Cost-Effectiveness of Small Molecule Drugs
Small molecule drugs are typically more cost-effective than biologic treatments, making them a preferred choice for both patients and healthcare systems, particularly in emerging markets. Their affordability helps improve access to essential treatments, driving market growth in countries with limited healthcare budgets. For instance, in regions such as India and Brazil, topical corticosteroids such as Hydrocortisone and Betamethasone continue to be widely prescribed for treating conditions such as eczema, acne, and psoriasis, primarily due to their lower costs compared to biologic alternatives such as anti-TNF drugs. This cost-effectiveness serves as a key driver for the growth of the Dermatology Small Molecule API Market, as it enables broader patient access and adoption, particularly in developing regions, fostering the market's expansion.
Opportunities
- Rise in Dermatological Conditions
The increasing prevalence of common skin conditions such as acne, eczema, psoriasis, and rosacea are driving the demand for small molecule treatments. As the global population seeks more effective and long-term solutions for these chronic dermatological issues, the need for small molecule APIs in dermatology continues to grow. For instance, acne vulgaris is estimated to affect 9.4% of the global population (source: Global Burden of Disease Study, 2019), representing a substantial patient base. This growing demand for treatments such as tretinoin and benzoyl peroxide highlights a clear market opportunity for small molecule drugs in dermatology. With the rising burden of skin diseases worldwide, the dermatology small molecule API market is positioned for significant expansion, creating numerous opportunities for pharmaceutical companies to develop and deliver effective solutions to a large and diverse patient population.
- Innovations in Drug Formulations
Advancements in drug delivery technologies, including sustained-release formulations, nano-drug delivery systems, and transdermal patches, are creating new opportunities for small molecule drugs in dermatology. These innovations enhance the efficacy of treatments, improve patient compliance, and minimize side effects. For instance, Tretinoin (Retin-A), a well-established small molecule used for acne treatment, is now offered in sustained-release formulations that enhance its therapeutic effectiveness while reducing skin irritation. These improvements align with the growing demand for more efficient, patient-friendly, and less irritating treatments. As drug delivery technologies continue to evolve, the dermatology small molecule API market is poised to benefit, offering new opportunities for pharmaceutical companies to address patient needs for more convenient, effective, and safer treatments.
Restraints/Challenges
- Regulatory and Approval Challenges
The dermatology small molecule API market faces regulatory hurdles that can delay product approvals, particularly for new formulations or indications. Regulatory agencies such as the FDA and EMA impose stringent requirements for clinical trials, efficacy, safety, and manufacturing standards, which can increase development timelines and costs. For instance, obtaining approval for topical drugs may require extensive clinical data to demonstrate efficacy in treating specific dermatological conditions such as acne, psoriasis, or eczema. For instance, Benzoyl Peroxide, a widely used topical treatment for acne, was initially subject to rigorous approval processes therefore, regulatory complexities are a significant restraint on the market,
- Competition from Biologic Therapies
The dermatology small molecule API market faces stiff competition from biologics, which offer superior efficacy for severe or treatment-resistant conditions such as moderate to severe psoriasis or atopic dermatitis. Biologic therapies, such as TNF inhibitors and monoclonal antibodies, often provide more targeted and potent effects, making them the preferred choice for complex skin disorders despite their higher costs. For instance, Dupilumab (Dupixent), a monoclonal antibody, has demonstrated superior efficacy in treating moderate to severe atopic dermatitis (AD) and is increasingly used over small molecule treatments due to its long-term benefits in managing chronic conditions. The growing preference for biologic therapies, which offer targeted treatment with fewer side effects, represents a major challenge for the small molecule API market in dermatology.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Dermatology Small Molecule API Market Scope
The market is segmented on the basis of drug class, application, route of administration, distribution channel, and end-use. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Anti-Infectives
- Corticosteroids
- Anti-Acne
- Calcineurin Inhibitors
- Retinoids
- Others
Application
- Acne
- Psoriasis
- Atopic Dermatitis
- Others
Route of Administration
- Oral
- Parenteral
- Topical
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
End-Use
- Hospitals
- Skin Clinics
- Others
Dermatology Small Molecule API Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug class, application, route of administration, distribution channel, and end-use as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is anticipated to maintain a substantial market share, driven by factors such as increasing healthcare expenditure, heightened awareness of skin diseases, and the robust presence of leading dermatology pharmaceutical companies. The growing incidence of dermatological conditions is a key factor expected to propel market growth in the region. For instance, a report from the World Allergy Organization Journal published in March 2023 highlighted that the prevalence of atopic dermatitis in the U.S. ranges from 4.9% to 10.2%. This high prevalence of dermatological diseases is expected to significantly drive the demand for dermatological therapeutics in the country.
Asia-Pacific is projected to experience the highest growth rate throughout the forecast period. The region has emerged as a key commercial hub for the dermatology industry, driven by factors such as the increasing prevalence of skin diseases, an aging population, and rising healthcare costs. Furthermore, various government initiatives and collaborations are actively working to enhance attention on dermatological concerns, contributing to the region’s growing market potential.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Dermatology Small Molecule API Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Dermatology Small Molecule API Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Arcutis Biotherapeutics, Inc. (U.S.)
- BIOCAD HK Ltd (China)
- Bristol-Myers Squibb Company (U.S.)
- Can-Fite (Israel)
- CELLTRION INC. (Republic of Korea)
- Coherus BioSciences (U.S.)
- DermBiont, Inc. (U.S.)
- Galectin Therapeutics, Inc. (U.S.)
- Galderma (Switzerland)
- ILTOO Pharma (France)
- GSK plc. (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- LEO Pharma A/S (Denmark)
- Pfizer Inc. (U.S.)
- Roivant Sciences Ltd. (U.K.)
- Sun Pharmaceutical Industries Ltd. (India)
- Viatris Inc. (U.S.)
Latest Developments in Dermatology Small Molecule API Market
- In October 2024, LEO Pharma released the financial highlights from first nine months of 2024, The company delivered revenue growth of 11% in constant exchange rates (CER). The dermatology portfolio saw revenue growth of 13%. It also included several key events around the globe such as the European Commission’s approval of Anzupgo (delgocitinib) cream for adults with moderate to severe chronic hand eczema (CHE)
- In October 2024, Organon announced the successful completion of its acquisition of Dermavant Sciences Ltd. from Roivant. Dermavant is a company dedicated to developing and commercializing innovative therapeutic solutions in immuno-dermatology. It included acquisition of VTAMA (tapinarof) cream, 1%, which is a novel nonbiologic, non-steroidal topical therapy approved by the U.S. Food and Drug Administration (FDA) for treatment of mild, moderate, and severe plaque psoriasis
- In August 2024, DermBiont, announced positive initial data from its ongoing open label, multi-center Phase 2a study for the treatment of basal cell carcinoma (BCC). The results showed that lesions which were treated with SM-020 1% gel BID (twice daily) for 28 days, with the primary endpoint based on percent change from baseline in greatest tumor diameter at week 6, showed a prompt (within days of starting treatment) and robust clinical response to the application of SM-020 1% gel, clearly demonstrating the disease modifying potential of SM-020
- In May 2024, Johnson & Johnson announced that it had reached a definitive agreement to acquire Proteologix, Inc., a privately-held biotechnology firm specializing in bispecific antibodies for immune-related diseases, for $850 million in cash, with the potential for additional milestone payments. Proteologix’s pipeline includes PX128, a bispecific antibody targeting IL-13 and TSLP, which is poised to enter Phase 1 development for the treatment of moderate to severe atopic dermatitis (AD) and moderate to severe asthma
- In March 2024, Amgen announced new, 52-week results from the Phase 3 SPROUT study examining the use of Otezla (apremilast) in children and adolescents aged 6 to 17 years with moderate to severe plaque psoriasis. Continued Otezla use resulted in sustained improvements in psoriasis severity and skin involvement in pa tients for up to one year
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.