Global Diabetic Macular Edema Steroids Market
Market Size in USD Billion
CAGR :
% 
 USD
5.52 Billion
USD
10.47 Billion
2024
2032
USD
5.52 Billion
USD
10.47 Billion
2024
2032
| 2025 –2032 | |
| USD 5.52 Billion | |
| USD 10.47 Billion | |
|
|
|
|
Diabetic Macular Edema Steroids Market Analysis
The global diabetic macular edema (DME) steroids market is driven by the rising prevalence of diabetic macular edema, a common complication of diabetes. According to the International Diabetes Federation (IDF), in 2023, an estimated 537 million adults (20-79 years) were living with diabetes worldwide, and approximately 10% of these individuals are at risk of developing DME. The condition predominantly affects patients with longer durations of diabetes, with studies indicating a 3% to 7% prevalence rate among those with type 2 diabetes. Corticosteroid-based therapies have emerged as effective treatments for reducing inflammation and fluid accumulation in the macula, particularly in cases unresponsive to anti-VEGF therapies. The market is further bolstered by advancements in intravitreal implant technologies, such as dexamethasone implants, which provide sustained drug delivery and improved visual outcomes for patients. Increasing awareness and screening programs in regions with high diabetes prevalence, such as Asia-Pacific and the Middle East, are expected to enhance diagnosis and treatment rates.
Diabetic Macular Edema Steroids Market Size
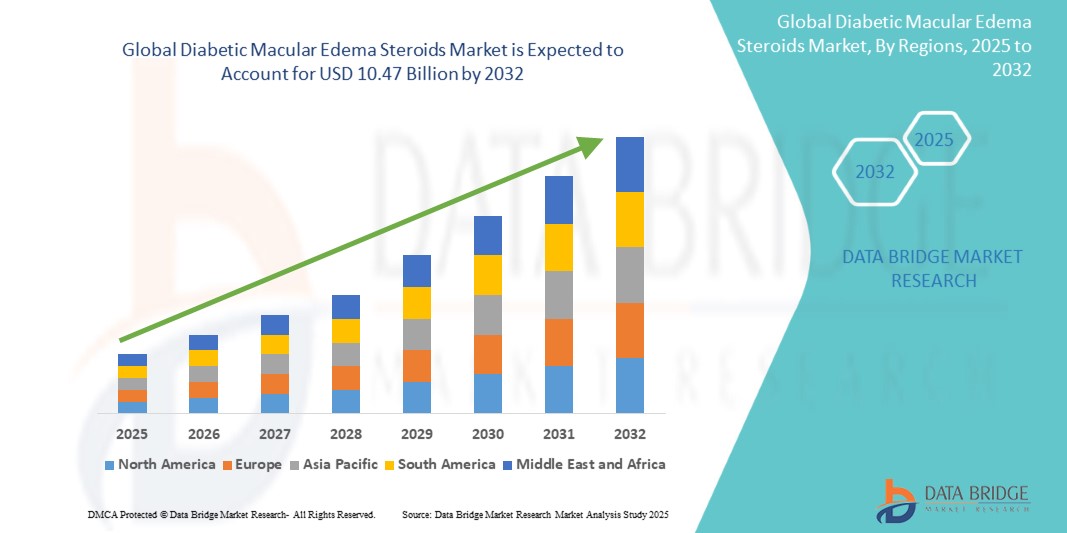
Global diabetic macular edema steroids market size was valued at USD 5.52 billion in 2024 and is projected to reach USD 10.47 billion by 2032, with a CAGR of 7.46% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Diabetic Macular Edema Steroids Market Trends
“Shift Towards Long-Acting Steroid Implants”
The preference for long-acting intravitreal steroid implants in the management of diabetic macular edema (DME) reflects a significant trend in the market. Implants such as dexamethasone and fluocinolone acetonide are designed to deliver corticosteroids steadily over an extended period, typically several months, reducing the need for frequent injections. This extended-release mechanism not only alleviates the burden on healthcare systems but also enhances the treatment experience for patients, who often face challenges in adhering to traditional, repetitive injection schedules. These implants address the inflammatory component of DME effectively while providing a practical solution for sustained therapeutic outcomes. Their adoption underscores the growing emphasis on innovative, patient-friendly solutions in ophthalmology.
Report Scope and Diabetic Macular Edema Steroids Market Segmentation
|
Attributes |
Diabetic Macular Edema Steroids Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
AbbVie (U.S.), Alimera Sciences (U.S.), Regeneron Pharmaceuticals (U.S.), Bausch + Lomb (Canada), F. Hoffmann-La Roche Ltd (Switzerland), Pfizer Inc. (U.S.), Santen Pharmaceutical Co., Ltd. (Japan), Novartis AG (Switzerland), Clearside Biomedical (U.S.), Kodiak Sciences Inc. (U.S.), Ocular Therapeutix, Inc. (U.S.), Eyepoint Pharmaceuticals (U.S.), Sanofi (France), Biogen Inc. (U.S.), Aerie Pharmaceuticals (U.S.), Graybug Vision, Inc. (U.S.), and Bayer AG (Germany) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Diabetic Macular Edema Steroids Market Definition
Diabetic Macular Edema (DME) Steroids are a class of corticosteroid medications used to treat Diabetic Macular Edema (DME), a complication of diabetic retinopathy where the macula, the central part of the retina responsible for sharp vision, swells due to fluid leakage from blood vessels. Steroids, such as dexamethasone and fluocinolone acetonide, help reduce inflammation, control fluid accumulation, and improve vision by decreasing swelling in the retina. These steroids are often administered through intravitreal injections or implants, providing targeted delivery to the affected area, offering a long-lasting effect compared to other treatment options.
Diabetic Macular Edema Steroids Market Dynamics
Drivers
- Increasing Prevalence of Diabetes
The increasing prevalence of diabetes, especially Type 2 diabetes, is a significant driver of the global diabetic macular edema (DME) steroids market. Diabetes is a leading cause of diabetic retinopathy, a condition that damages the blood vessels in the retina. As the global incidence of diabetes rises, so does the prevalence of diabetic retinopathy and its serious complication, diabetic macular edema (DME), which causes fluid buildup in the retina and can lead to vision loss. The growing number of diabetes cases, particularly in developing countries, is driving the need for effective treatment options. Steroid-based therapies, such as dexamethasone and fluocinolone acetonide, are commonly used to treat DME by reducing inflammation and controlling fluid leakage in the retina. As DME becomes more widespread, the demand for these steroids to manage the condition and prevent further vision impairment continues to rise, contributing to market growth.
- Advancements in Steroid Delivery Systems
Advancements in steroid delivery systems have played a crucial role in the growth of the global diabetic macular edema (DME) steroids market. Traditional steroid treatments for DME typically require frequent intravitreal injections, which can be burdensome for patients and lead to lower adherence. However, the development of long-acting intravitreal implants, such as dexamethasone and fluocinolone acetonide implants, has significantly improved the convenience of DME treatment. These implants provide sustained drug release over several months, reducing the need for repeated injections and minimizing patient discomfort. This shift towards sustained-release formulations has not only enhanced patient compliance but also improved the efficacy of DME management by maintaining consistent drug levels in the retina. As a result, these advancements have led to greater adoption of steroid-based therapies, making them a preferred choice in treating diabetic macular edema and contributing to the market's expansion.
Opportunities
- Combination Therapies with Other Treatments
Combination therapies that integrate steroids with other treatments, such as anti-VEGF (vascular endothelial growth factor) drugs or laser therapies, offer a comprehensive approach to managing Diabetic Macular Edema (DME). DME is a complex condition involving both inflammation and the growth of abnormal blood vessels that leak fluid into the retina, causing swelling. Steroids help to reduce inflammation, while anti-VEGF drugs target and inhibit the abnormal blood vessel growth. By combining these therapies, it is possible to address both key drivers of the disease simultaneously, improving overall treatment outcomes. Additionally, laser treatments, which focus on stabilizing the retina and reducing fluid accumulation, complement the effects of steroids and anti-VEGF drugs. This multifaceted approach can lead to enhanced patient outcomes, such as improved vision and reduced disease progression. As a result, the growing adoption of combination therapies represents a significant opportunity for expanding the market for DME steroids.
- Growing Aging Population
The global aging population, particularly in developed countries, presents a significant opportunity for the DME steroids market. As people age, the risk of developing diabetes and its complications, including Diabetic Macular Edema (DME), increases. Age-related factors such as reduced metabolism and weakened immune systems contribute to a higher prevalence of diabetes-related eye conditions. As the elderly population grows, so does the demand for effective treatments to manage diabetic retinopathy and DME, which can lead to vision loss if left untreated. The increasing need for therapies that are both effective and convenient for elderly patients offers a strong market opportunity for long-acting steroid implants and other therapeutic innovations. These treatments, which provide sustained drug delivery and reduce the frequency of injections, are especially beneficial for older patients who may face challenges with regular treatments. This demographic trend is expected to significantly expand the demand for DME steroids in the market.
Restraints/Challenges
- High Cost of Treatment
The high cost of treatment is a significant restraint in the Global Diabetic Macular Edema (DME) Steroids Market, particularly in developing countries where healthcare resources are limited. Steroid-based treatments for DME, such as intravitreal implants and other therapies, often require specialized medical professionals and facilities for administration, contributing to their high cost. The need for regular treatments and follow-ups further increases the financial burden on patients. In countries with limited healthcare budgets, this can make it difficult for many individuals to access these treatments, resulting in a lower adoption of steroid therapies. Additionally, the availability of cost-effective alternatives, such as anti-VEGF drugs, may make patients and healthcare providers opt for more affordable options. As a result, the high cost of steroid-based DME treatments may restrict patient access to effective care, thereby limiting the market's growth in these regions.
- Regulatory and Safety Concerns
Regulatory and safety concerns present a significant challenge for the Global Diabetic Macular Edema (DME) Steroids Market. Steroid treatments, particularly intravitreal injections and implants, face strict regulatory oversight due to potential side effects, such as increased intraocular pressure, glaucoma, and cataracts. Any changes in regulatory policies or growing concerns about the long-term safety of steroids in treating DME could impact their approval or usage. Moreover, healthcare professionals may be cautious in prescribing steroids, especially for patients with pre-existing conditions such as glaucoma, where steroids can worsen the situation. These factors may slow the adoption of steroid therapies, as physicians may prefer safer alternatives such as anti-VEGF drugs. The evolving regulatory landscape and safety issues make it challenging for the steroid-based DME treatments market to expand, potentially limiting patient access and treatment options.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Diabetic Macular Edema Steroids Market Scope
The market is segmented on the basis of drug type, route of administration, age group, gender, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Type
- Intravitreal implants
- Injectable corticosteroids
Route of Administration
- Intravitreal
- Periocular
Age Group
- Below 40 years
- 40–60 years
- Above 60 years
Gender
- Male
- Female
End User
- Healthcare Providers
- Research Organizations
- Patients
Distribution Channel
- Hospitals
- Specialty Clinics
- Online Pharmacies
- Retail Pharmacies
Diabetic Macular Edema Steroids Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, drug type, route of administration, age group, gender, end user, and distribution channel as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its highly advanced healthcare infrastructure, which supports the widespread adoption of cutting-edge technologies. The region's high prevalence of chronic conditions, particularly diabetes, creates a strong demand for improved diagnostic tools, including AI-driven solutions, to enhance early detection and management.
Asia-Pacific is expected to be the fastest growing due to several key factors. The rising prevalence of diabetes, along with other chronic diseases, is placing increasing pressure on healthcare systems, driving the need for more efficient and accurate diagnostic solutions such as AI-powered pathology tools.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Diabetic Macular Edema Steroids Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Diabetic Macular Edema Steroids Market Leaders Operating in the Market Are:
- AbbVie (U.S.)
- Alimera Sciences (U.S.)
- Regeneron Pharmaceuticals (U.S.)
- Bausch + Lomb (Canada)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Pfizer Inc. (U.S.)
- Santen Pharmaceutical Co., Ltd. (Japan)
- Novartis AG (Switzerland)
- Clearside Biomedical (U.S.)
- Kodiak Sciences Inc. (U.S.)
- Ocular Therapeutix, Inc. (U.S.)
- Eyepoint Pharmaceuticals (U.S.)
- Sanofi (France)
- Biogen Inc. (U.S.)
- Aerie Pharmaceuticals (U.S.)
- Graybug Vision, Inc. (U.S.)
- Bayer AG (Germany)
Latest Developments in Diabetic Macular Edema Steroids Market
- In October 2024, EyePoint Pharmaceuticals, Inc. announced positive 16-week interim data from its ongoing Phase 2 VERONA trial for DURAVYU, a sustained delivery therapy with vorolanib, a selective tyrosine kinase inhibitor, formulated in the bioerodible Durasert E™ for diabetic macular edema (DME) patients. This positive outcome strengthens the company’s position in the DME treatment market and advances the potential for DURAVYU as a key therapy
- In October 2024, Oculis Holding AG announced that David Eichenbaum, M.D. will present an update on the DIAMOND Phase 3 program for OCS-01, a high-concentration dexamethasone eye drop formulation, at Innovate Retina for diabetic macular edema (DME). This presentation will highlight the progress of OCS-01 and enhance the company's visibility in the DME market
- In September 2024, Merck and EyeBio announced the launch of the Phase 2b/3 BRUNELLO trial to evaluate Restoret (MK-3000, formerly EYE103) for treating diabetic macular edema (DME). This trial advances Restoret as a potential treatment, strengthening Merck's position in the DME market
- In July 2024, Genentech, a member of the Roche Group, announced two-year data from the Phase III Pagoda and Pavilion studies evaluating Susvimo® (ranibizumab injection) 100 mg/mL for treating diabetic macular edema (DME) and diabetic retinopathy (DR). This data reinforces Susvimo® as a promising long-term treatment option, enhancing the company's position in the diabetic eye disease market
- In May 2024, Merck and Eyebiotech Limited announced a definitive agreement for Merck, through a subsidiary, to acquire EyeBio. This acquisition strengthens Merck’s eye care portfolio and enhances its capabilities in the treatment of ocular diseases
- In January 2024, EyePoint Pharmaceuticals, Inc. announced the dosing of the first patient in the Phase 2 VERONA trial of EYP-1901 for diabetic macular edema (DME). EYP-1901 is a sustained delivery therapy with vorolanib, a selective tyrosine kinase inhibitor, formulated in bioerodible Durasert E. This milestone positions the company to advance EYP-1901 as a potential treatment for DME, enhancing its pipeline and market potential.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.