Global Digital Twin Technology For Medical Devices Market
Market Size in USD Million
CAGR :
% 
 USD
411.64 Million
USD
1,406.39 Million
2024
2032
USD
411.64 Million
USD
1,406.39 Million
2024
2032
| 2025 –2032 | |
| USD 411.64 Million | |
| USD 1,406.39 Million | |
|
|
|
|
Digital Twin Technology for Medical Devices Market Size
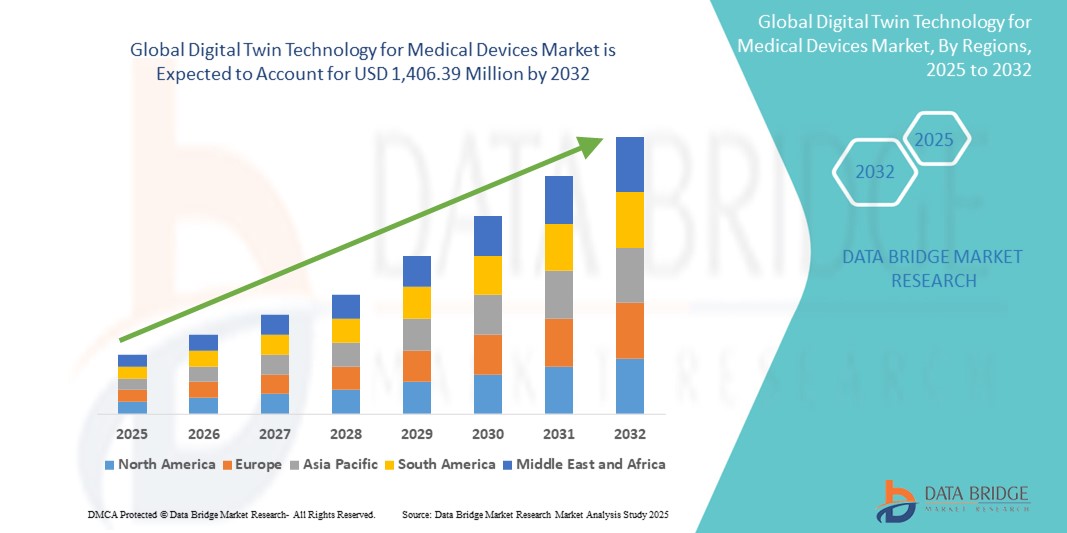
- The global digital twin technology for medical devices market size was valued at USD 411.64 million in 2024 and is expected to reach USD 1,406.39 million by 2032, at a CAGR of 16.60% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within connected medical devices and healthcare digital infrastructure, leading to increased digitalization across diagnostic, therapeutic, and monitoring applications
- Furthermore, rising demand for real-time data, predictive analytics, and personalized healthcare is establishing digital twin technology as a transformative tool in modern medicine. These converging factors are accelerating the uptake of digital twin technology for medical devices solutions, thereby significantly boosting the industry's growth
Digital Twin Technology for Medical Devices Market Analysis
- Digital twin technology, which involves creating virtual replicas of physical medical devices and systems, is becoming an increasingly vital component of modern healthcare innovation. It enhances predictive maintenance, personalized treatment planning, and product lifecycle management by enabling real-time simulation, monitoring, and analytics
- The rising demand for digital twin solutions in the medical device sector is primarily fueled by increasing adoption of connected healthcare technologies, growing emphasis on precision medicine, and the need to optimize device performance, reduce operational costs, and enhance patient outcomes
- North America dominated the digital twin technology for medical devices market with the largest revenue share of 41.8% in 2024, driven by a strong digital infrastructure, rapid adoption of advanced healthcare technologies, and substantial investments in R&D by both established medical device manufacturers and startups
- Asia-Pacific is expected to be the fastest-growing region in the digital twin technology for medical devices market, registering a CAGR of 22.9% during the forecast period. This growth is attributed to increasing urbanization, expanding healthcare IT investments, and rising demand for cost-effective, technology-enabled medical solutions across countries such as China, India, and Japan
- The product digital twin segment dominated the digital twin technology for medical devices market with the largest revenue share of 45.6% in 2024, owing to its widespread use in simulating real-world device behavior during design and testing phases
Report Scope and Digital Twin Technology for Medical Devices Market Segmentation
|
Attributes |
Digital Twin Technology for Medical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Digital Twin Technology for Medical Devices Market Trends
“Real-Time Simulation and Personalized Healthcare Are Reshaping Medical Device Development”
- A significant and rapidly advancing trend in the global digital twin technology for medical devices market is the growing use of real-time simulation tools that enable the creation of virtual replicas of medical devices for testing, optimization, and lifecycle management. These digital counterparts provide clinicians and manufacturers with critical data on performance, usage patterns, and potential failure points—without needing to rely solely on physical prototypes
- For instance, Siemens Healthineers is leveraging digital twin platforms to model imaging systems and predict maintenance needs, reducing downtime and enhancing device reliability. Similarly, GE Healthcare uses digital twin technology to optimize the design and functionality of ventilators and other life-critical equipment by simulating different usage environments and patient profiles
- The technology also supports highly personalized patient care. By integrating patient-specific data, digital twins allow simulation of physiological responses to medical interventions, such as cardiac implants or insulin pumps, enabling clinicians to tailor treatment strategies more accurately. This predictive capability enhances both patient safety and clinical outcomes
- In addition, digital twin models play a vital role in post-market surveillance, enabling continuous performance monitoring and early detection of malfunctions or inefficiencies in deployed devices. This proactive approach aids regulatory compliance and strengthens risk management in healthcare settings
- Integration with hospital IT systems and cloud infrastructure further allows seamless data exchange, remote diagnostics, and collaborative treatment planning. These interoperable platforms are driving adoption in both developed and emerging healthcare markets
- As a result, manufacturers and healthcare providers are increasingly investing in digital twin solutions to accelerate R&D, improve patient outcomes, and reduce costs across the medical device lifecycle. This trend is poised to transform not only product development but also the entire healthcare delivery ecosystem
Digital Twin Technology for Medical Devices Market Dynamics
Driver
“Growing Need Due to Rising Healthcare Digitization and Predictive Care Models”
- The increasing emphasis on personalized medicine and real-time patient monitoring is a major driver fueling the demand for digital twin technology in the medical devices market. As hospitals and medical manufacturers seek greater operational efficiency and predictive maintenance, digital twin systems are emerging as vital tools in modern healthcare infrastructure
- For instance, in March 2024, Siemens Healthineers announced enhancements in its digital twin platform to simulate cardiovascular procedures using patient-specific data, reducing the risk of surgical complications. Such innovations are expected to accelerate the growth of the digital twin technology for medical devices industry during the forecast period
- Digital twin models offer precise, virtual replicas of medical devices that help simulate behavior under varying physiological conditions. This allows healthcare providers and device manufacturers to anticipate device failure, optimize performance, and tailor therapies to individual patients
- Furthermore, rising adoption of Internet of Medical Things (IoMT), integration of AI/ML algorithms, and increasing demand for remote diagnostics and connected care environments are making digital twins an integral component in next-generation medical technologies
- In both hospitals and manufacturing units, these systems enhance R&D productivity, reduce the need for physical prototypes, and ensure regulatory compliance by offering traceable virtual validation models. The synergy between cloud computing, big data, and 3D visualization is further strengthening the role of digital twins in medical device development and clinical practice
Restraint/Challenge
“Concerns Regarding Cybersecurity and High Initial Costs”
- Despite its transformative potential, the widespread adoption of digital twin technology in the medical devices sector faces substantial challenges, particularly concerning patient data privacy, system integration complexity, and high implementation expenses
- These technologies rely on vast volumes of patient-specific and device-generated data, raising concerns about data security and compliance with regulations such as HIPAA (U.S.) and GDPR (EU). Ensuring encryption, access control, and ethical data handling remains a significant barrier for healthcare providers and manufacturers alike
- In addition, the lack of standardization across medical systems and device platforms limits the seamless integration of digital twins with existing EHR systems, imaging tools, and analytics dashboards. This interoperability gap can delay deployment timelines and increase the cost of customization
- High initial investment, which includes cloud infrastructure, data modeling tools, and real-time analytics engines, is a further deterrent for smaller hospitals and startups. While digital twin deployment yields long-term operational and clinical benefits, the upfront cost remains a key barrier to entry, especially in emerging economies
- To ensure market scalability, vendors are increasingly focusing on modular digital twin platforms, open standards for data sharing, and secure APIs. In addition, collaborative efforts between med-tech companies, healthcare institutions, and regulatory agencies will be critical in building trust and accelerating safe, cost-effective adoption of digital twin technologies in the healthcare ecosystem
Digital Twin Technology for Medical Devices Market Scope
The market is segmented on the basis of four notable segments based on type, technology, application, and end user.
• By Type
On the basis of type, the digital twin technology for medical devices market is segmented into product digital twin, process digital twin, and system digital twin. The product digital twin segment dominated the market with the largest revenue share of 45.6% in 2024, owing to its widespread use in simulating real-world device behavior during design and testing phases.
The System Digital Twin segment is projected to register the fastest CAGR of 18.4% from 2025 to 2032, driven by growing adoption for simulating entire healthcare operations, improving patient management and hospital performance.
• By Technology
On the basis of technology, the digital twin technology for medical devices market is segmented into IoT, AI & Machine Learning, Big Data Analytics, Blockchain, and Others. The IoT segment held the largest market share of 36.3% in 2024, fueled by increasing connectivity between physical medical devices and digital platforms.
The AI & Machine Learning segment is expected to witness the fastest CAGR of 20.2% during the forecast period, owing to its potential in predictive analytics, anomaly detection, and treatment simulation.
• By Application
On the basis of application, the digital twin technology for medical devices market is segmented into Diagnosis Support, Personalized Treatment, Surgical Planning, Remote Monitoring, Asset and Process Management, and Others. The Personalized Treatment segment accounted for the largest market revenue share of 33.7% in 2024, as digital twins enable simulation-based customization of care pathways.
The Remote Monitoring segment is forecasted to grow at the highest CAGR of 21.5% from 2025 to 2032, supported by increasing demand for home-based healthcare and chronic disease monitoring.
• By End User
On the basis of end user, the digital twin technology for medical devices market is segmented into Hospitals and Clinics, Research and Academic Institutes, Medical Device Companies, and Others. The Hospitals and Clinics segment held the largest revenue share of 48.1% in 2024, due to the widespread use of digital twins for improving care delivery and operational efficiency.
The Medical Device Companies segment is expected to expand at the highest CAGR of 19.7% during the forecast period, driven by increased use in device design, regulatory testing, and lifecycle monitoring.
Digital Twin Technology for Medical Devices Market Regional Analysis
- North America dominated the digital twin technology for medical devices market with the largest revenue share of 41.8% in 2024, driven by increasing adoption of advanced healthcare technologies, growing investments in digital infrastructure, and a strong presence of key market players
- The region’s focus on improving patient outcomes and operational efficiencies has accelerated the integration of digital twin technologies across hospitals, clinics, and medical device manufacturers
- The increasing reliance on simulation, predictive modeling, and virtual monitoring further supports the adoption of digital twin solutions in various stages of medical device development and clinical deployment
U.S. Digital Twin Technology for Medical Devices Market Insight
The U.S. digital twin technology for medical devices market captured the largest revenue share of 76.2% in 2024 within North America, owing to the country’s leadership in medical device innovation, robust R&D ecosystem, and growing demand for personalized healthcare. Consumers are increasingly prioritizing real-time monitoring and predictive diagnostics, further propelling market growth. Additionally, the rapid adoption of technologies such as IoT, cloud computing, and advanced analytics has significantly contributed to the expansion of digital twin applications in medical settings.
Europe Digital Twin Technology for Medical Devices Market Insight
The Europe digital twin technology for medical devices market is projected to expand at a substantial CAGR throughout the forecast period. The market is driven by stringent healthcare regulations, increased funding for healthcare innovation, and growing demand for precision medicine. The integration of digital twins for improving treatment accuracy, minimizing errors, and streamlining operations is gaining momentum across healthcare providers and research institutions in the region.
U.K. Digital Twin Technology for Medical Devices Market Insight
The U.K. digital twin technology for medical devices market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by increasing investments in digital health, growing focus on patient-centric care, and expanding use of simulation in surgical planning and clinical trials. The nation's supportive regulatory environment and the presence of key medtech companies contribute to the growing demand for digital twin solutions in healthcare.
Germany Digital Twin Technology for Medical Devices Market Insight
The Germany digital twin technology for medical devices market is expected to expand at a considerable CAGR during the forecast period, supported by the country’s strong engineering and innovation ecosystem. Germany’s healthcare institutions are leveraging digital twin tools for improving diagnostics, optimizing medical device performance, and enhancing operational workflows in hospitals. The focus on sustainability and digital transformation further aligns with market growth.
Asia-Pacific Digital Twin Technology for Medical Devices Market Insight
The Asia-Pacific digital twin technology for medical devices market is poised to grow at the fastest CAGR of 22.9% during the forecast period of 2025 to 2032. This rapid growth is driven by rising healthcare investments, increasing government support for smart hospital initiatives, and growing awareness of digital healthcare technologies across countries such as China, Japan, India, and South Korea. As the region becomes a manufacturing hub for digital twin systems and components, affordability and innovation are enabling wider adoption.
Japan Digital Twin Technology for Medical Devices Market Insight
The Japan digital twin technology for medical devices market is forecasted to grow at a notable CAGR during the forecast period, fueled by the country’s high-tech infrastructure, aging population, and increased focus on precision and preventive care. Medical device companies and healthcare providers are utilizing digital twin models to simulate patient outcomes, plan surgeries, and manage chronic conditions more effectively.
China Digital Twin Technology for Medical Devices Market Insight
The China digital twin technology for medical devices market accounted for the largest revenue share of 44.9% in Asia-Pacific in 2024, propelled by the government’s push for digital health transformation, rapid urbanization, and rising healthcare spending. China’s strong domestic medtech manufacturing base, along with increasing partnerships between tech companies and healthcare institutions, is significantly contributing to the expansion of digital twin applications in diagnostics, treatment planning, and patient management.
Digital Twin Technology for Medical Devices Market Share
The digital twin technology for medical devices industry is primarily led by well-established companies, including:
- Siemens Healthineers AG (Germany)
- GE HealthCare (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Dassault Systèmes SE (France)
- IBM Corporation (U.S.)
- Microsoft Corporation (U.S.)
- Oracle (U.S.)
- ANSYS, Inc. (U.S.)
- Twin Health (U.S.)
- Atos SE (France)
- Altair Engineering, Inc. (U.S.)
- SAP SE (Germany)
- PTC Inc. (U.S.)
- PHYSNA INC (U.S.)
- Predixion Software (U.S.)
- Rescale Inc. (U.S.)
- Medtronic (Ireland)
- Boston Scientific Corporation (U.S.)
- Siemens (Germany)
- General Electric Company (U.S.)
Latest Developments in Global Digital Twin Technology for Medical Devices Market
- In April 2023, Siemens Healthineers announced the expansion of its digital twin capabilities in medical imaging by integrating its AI-powered simulation tools with clinical workflow systems. This initiative aims to enhance diagnostic accuracy and operational efficiency in hospitals by replicating real-time patient and equipment data. The move demonstrates Siemens' commitment to transforming medical imaging and diagnostics through advanced digital twin technology, further solidifying its position in the global Digital Twin Technology for Medical Devices market
- In March 2023, GE HealthCare unveiled its new AI-enabled digital twin platform at the HIMSS Global Health Conference. The platform, designed to model patient-specific conditions and simulate treatment outcomes, is intended for use in cardiovascular and oncology diagnostics. This innovation marks a significant advancement in precision medicine, empowering clinicians to make data-driven, personalized treatment decisions with higher confidence
- In March 2023, Philips collaborated with a consortium of academic institutions and medical device manufacturers to launch a pilot project using digital twins for remote surgical planning and training. The initiative utilizes real-time physiological data and 3D anatomical modeling to prepare and educate surgeons before complex procedures. This partnership reflects a growing emphasis on simulation-based clinical training and the evolving role of digital twins in medical education and planning
- In February 2023, Medtronic announced a strategic investment in a startup focused on digital twin modeling of chronic disease progression, particularly in diabetes and cardiovascular care. The collaboration will allow Medtronic to enhance its portfolio of smart medical devices with predictive analytics, enabling real-time monitoring and intervention for patients. This reflects a broader industry trend of integrating digital twins with wearable health technologies
- In January 2023, Dassault Systèmes introduced the next generation of its “Living Heart” digital twin project, expanding its capabilities to include congenital heart disease and pediatric care. The platform uses multi-physics simulation to create virtual models of patient-specific cardiac conditions, assisting clinicians in treatment planning and device development. This development underlines the potential of digital twin technology in advancing personalized medicine and regulatory science in the medical device industry
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.