Global Diphtheria Tetanus And Pertussis Vaccine Market
Market Size in USD Billion
CAGR :
% 
 USD
6.01 Billion
USD
9.75 Billion
2024
2032
USD
6.01 Billion
USD
9.75 Billion
2024
2032
| 2025 –2032 | |
| USD 6.01 Billion | |
| USD 9.75 Billion | |
|
|
|
|
Diphtheria, Tetanus and Pertussis Vaccine Market Analysis
The Diphtheria, Tetanus, and Pertussis (DTP) vaccine market is experiencing steady growth, driven by increasing awareness of the importance of immunization and the rising prevalence of infectious diseases. Government initiatives and vaccination programs worldwide are playing a crucial role in boosting the adoption of these vaccines, especially in low- and middle-income countries where access to healthcare might be limited. The rising focus on maternal and child health is also pushing the demand for DTP vaccines.
Advancements in vaccine technology, such as the development of vaccine combinations, have made immunization more convenient and cost-effective. This has further contributed to the growing adoption of these vaccines. The increasing number of government-funded vaccination programs, coupled with the growing healthcare expenditure, continues to support the market growth.
In addition, the growing number of awareness campaigns about vaccine-preventable diseases is positively influencing market expansion. However, challenges such as vaccine hesitancy, particularly in developed countries, and concerns over vaccine safety and efficacy, could impact the market's growth. Moreover, the availability of alternative vaccine types and competition among vaccine manufacturers could slightly slow down-market growth. Despite these challenges, the DTP vaccine market remains poised for steady growth, fueled by an ongoing focus on public health initiatives.
Diphtheria, Tetanus and Pertussis Vaccine Market Size
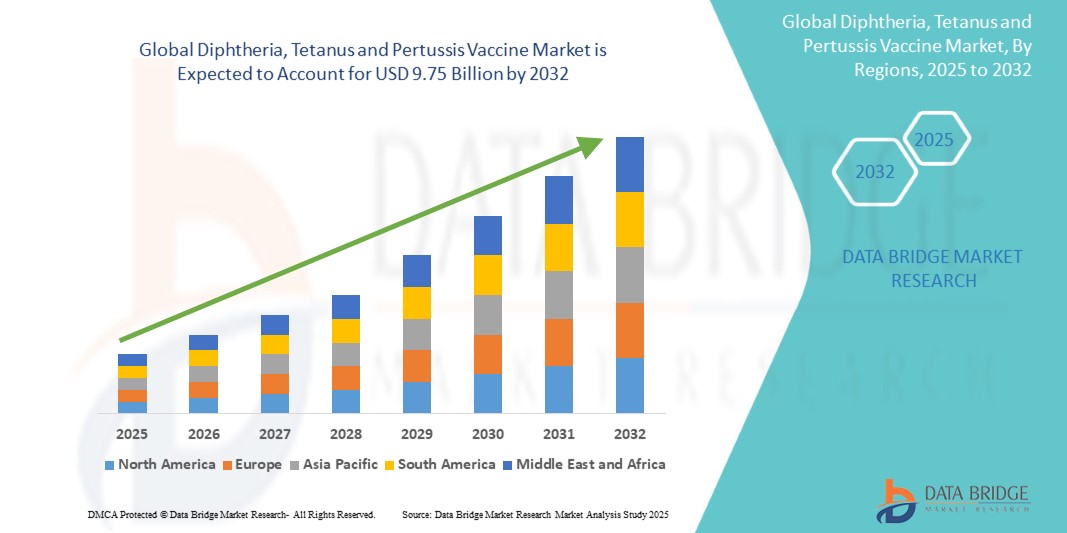
The global diphtheria, tetanus and pertussis vaccine market size was valued at USD 6.01 billion in 2024 and is projected to reach USD 9.75 billion by 2032, with a CAGR of 6.23% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Diphtheria, Tetanus and Pertussis Vaccine Market Trends
“Rising Adoption of Combination Vaccines”
A prominent trend in the diphtheria, tetanus, and pertussis (DTP) vaccine market is the rising adoption of combination vaccines. These vaccines, which combine DTP with other immunizations such as polio or hepatitis B, are becoming increasingly popular due to their ability to reduce the number of injections needed for children. This trend is driven by the demand for more streamlined vaccination schedules, improving convenience for both healthcare providers and patients. Combination vaccines also enhance patient compliance, as fewer visits to healthcare facilities are required. For governments and health organizations, these vaccines offer cost-effective solutions by reducing logistical expenses associated with multiple separate vaccines. In addition, the growing focus on reducing vaccine-preventable diseases in low- and middle-income countries has accelerated the demand for these combined solutions, further driving market growth. This trend reflects broader efforts to improve vaccination coverage and make immunization programs more efficient.
Report Scope and Diphtheria, Tetanus and Pertussis Vaccine Market Segmentation
|
Attributes |
Diphtheria, Tetanus and Pertussis Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
AJ Vaccines A/S (Denmark), Astellas Pharma Inc. (Japan), BioNet-Asia (Thailand), Biological E Limited (India), Bio Farma (Indonesia), Dynavax Technologies Corporation (U.S.), GSK plc. (UK), Johnson & Johnson Services, Inc. (U.S.), Incepta Pharmaceuticals Ltd. (Bangladesh), MassBiologics (U.S.), Meiji Holdings Co., Ltd. (Japan), Mitsubishi Chemical Group Corporation (Japan), Merck & Co., Inc. (U.S.), Pfizer Inc. (U.S.), Panacea Biotec (India), Sanofi (France), SINOVAC (China), Serum Institute of India Pvt. Ltd. (India), Takeda Pharmaceutical Company Limited (Japan), VBI Vaccines Inc (U.S.) and Walvax Biotechnology Co., Ltd. (China) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Diphtheria, Tetanus and Pertussis Vaccine Market Definition
The Diphtheria, Tetanus, and Pertussis (DTP) vaccine is a combination vaccine designed to protect individuals from three serious bacterial infections: diphtheria, tetanus, and pertussis. Diphtheria is a respiratory illness that can cause throat inflammation, breathing problems, and heart damage. Tetanus affects the nervous system, causing painful muscle stiffness and spasms, usually after a wound becomes contaminated. Pertussis, commonly known as whooping cough, is a highly contagious respiratory disease marked by severe coughing fits, which can be especially dangerous for infants. The DTP vaccine is typically administered during childhood as part of routine immunization programs, providing immunity to these diseases without causing the infections themselves. By stimulating the immune system to produce antibodies, the vaccine offers long-term protection, helping to reduce the spread of these potentially life-threatening diseases.
Diphtheria, Tetanus and Pertussis Vaccine Market Dynamics
Drivers
- Government Vaccination Programs
Government-led immunization campaigns play a crucial role in driving the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. Countries worldwide, especially those in low- and middle-income regions, have integrated the DTP vaccine into their national vaccination schedules. For instance, the Global Alliance for Vaccines and Immunization (GAVI) has been instrumental in providing DTP vaccines to underserved populations, helping increase immunization rates. National initiatives, such as the Expanded Programme on Immunization (EPI) in India, offer vaccines for free or at a low cost to ensure widespread coverage. These government efforts not only provide accessibility but also foster public trust in vaccines. As a result, governments' investments in immunization programs drive steady demand for DTP vaccines, contributing significantly to market growth.
- Technological Advancements in Vaccine Development
Technological advancements in vaccine development have become a significant driver of the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. Innovations such as the development of combination vaccines have made immunization more efficient and convenient. For instance, vaccines that combine DTP with other immunizations, such as polio or hepatitis B, are gaining popularity because they reduce the number of injections needed, which improves patient compliance, especially among children. Advances in vaccine formulation and delivery methods, such as improved adjuvants (substances that enhance the body’s immune response), have also increased the effectiveness and safety of DTP vaccines. These technological improvements help reduce vaccine-related side effects, which contributes to higher adoption rates. In addition, the development of more stable and cost-effective vaccines has facilitated broader distribution in underserved regions. These technological advancements make vaccines more accessible, safer, and easier to administer, significantly contributing to market growth.
Opportunities
- Expansion in Emerging Markets
Emerging markets, particularly in Asia and Africa, present significant growth opportunities for the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. As these regions continue to improve healthcare infrastructure, the demand for vaccines is on the rise, driven by government initiatives and international aid organizations. For instance, the World Health Organization’s efforts in sub-Saharan Africa to increase vaccine coverage are helping to reduce the burden of vaccine-preventable diseases. With an increasing focus on improving maternal and child health, countries such as India, Nigeria, and Indonesia are ramping up vaccination programs, creating a substantial market for DTP vaccines. In addition, GAVI’s involvement in making vaccines more affordable in these regions further enhances accessibility. This growth in emerging markets boosts global demand for DTP vaccines, presenting significant opportunities for manufacturers to expand their reach and contribute to market growth.
- Development of New Vaccine Formulations and Adjuvants
Ongoing research and development in vaccine formulations and adjuvants present a key opportunity for the DTP vaccine market. The introduction of more effective and safer versions of the DTP vaccine, including those with improved adjuvants (substances that boost immune response), can increase vaccine efficacy and reduce side effects. For instance, the development of acellular pertussis vaccines, which have fewer side effects compared to whole-cell vaccines, has contributed to broader acceptance of the vaccine. In addition, advancements in combination vaccines that incorporate DTP along with other immunizations, such as the MMR vaccine (measles, mumps, rubella), make vaccination more efficient. These innovations not only increase the attractiveness of DTP vaccines but also improve patient adherence to vaccination schedules. The introduction of new, more efficient formulations enhances demand, driving sustained growth and expanding market opportunities for DTP vaccine manufacturers.
Restraints/Challenges
- Vaccine Hesitancy and Misinformation
Vaccine hesitancy remains a significant restraint in the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. Misinformation and mistrust surrounding vaccines, often fueled by social media, have led to growing resistance in certain regions, particularly in developed countries. For instance, reports linking vaccines to autism, which have been widely debunked, have contributed to fear and refusal among parents to vaccinate their children. This hesitancy can result in lower immunization rates, creating an environment where diseases such as pertussis resurge. Even though public health campaigns are combating misinformation, it continues to hinder full vaccine adoption. In some cases, lower vaccination rates lead to outbreaks, increasing the need for more aggressive health interventions. This vaccine hesitancy limits the full market potential by reducing vaccine uptake, which can slow overall growth and impede progress toward herd immunity.
- Supply Chain and Distribution Issues
One of the major challenges in the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market is the complex supply chain and distribution issues, especially in low-income and rural areas. Vaccines require specific storage conditions (such as refrigeration), which can be difficult to maintain in remote or underserved regions. For instance, during the COVID-19 pandemic, supply chain disruptions highlighted vulnerabilities in vaccine distribution systems, which were also seen with routine vaccines such as DTP. These logistical challenges can delay vaccine delivery, resulting in missed vaccination schedules and limited access in hard-to-reach areas. Furthermore, irregular distribution and storage failures can reduce vaccine efficacy and affect public health outcomes. Supply chain challenges hinder the timely availability of vaccines, limiting market penetration and slowing overall growth, especially in developing regions.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Diphtheria, Tetanus and Pertussis Vaccine Market Scope
The market is segmented on the basis of product type, age group, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- DTaP vaccine
- TD vaccine
- Tdap vaccine
Age Group
- Adult
- Pediatrics
End User
- Hospitals
- Clinics and Vaccination Centers
Diphtheria, Tetanus and Pertussis Vaccine Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product type, age group, and end user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. This is primarily due to advanced healthcare infrastructure, well-established vaccination programs, and high immunization rates in countries such as the United States and Canada. Governments in these countries heavily invest in public health initiatives, ensuring widespread access to vaccines. The region also benefits from strong support for maternal and child health, as well as continuous research and development in vaccine technology, contributing to the availability of more effective and safer vaccines.
Asia-Pacific is expected to exhibit the highest growth rate in the Diphtheria, Tetanus, and Pertussis (DTP) vaccine market. This growth is driven by factors such as rapidly improving healthcare infrastructure, increasing government investments in immunization programs, and rising awareness about vaccine-preventable diseases. Countries such as India, China, and Indonesia are ramping up their efforts to improve vaccination coverage, particularly in rural and underserved areas.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Diphtheria, Tetanus and Pertussis Vaccine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Diphtheria, Tetanus and Pertussis Vaccine Market Leaders Operating in the Market Are:
- AJ Vaccines A/S (Denmark)
- Astellas Pharma Inc. (Japan)
- BioNet-Asia (Thailand)
- Biological E Limited (India)
- Bio Farma (Indonesia)
- Dynavax Technologies Corporation (U.S.)
- GSK plc. (UK)
- Johnson & Johnson Services, Inc. (U.S.)
- Incepta Pharmaceuticals Ltd. (Bangladesh)
- MassBiologics (U.S.)
- Meiji Holdings Co., Ltd. (Japan)
- Mitsubishi Chemical Group Corporation (Japan)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Panacea Biotec (India)
- Sanofi (France)
- SINOVAC (China)
- Serum Institute of India Pvt. Ltd. (India)
- Takeda Pharmaceutical Company Limited (Japan)
- VBI Vaccines Inc (U.S.)
- Walvax Biotechnology Co., Ltd. (China)
Latest Developments in Diphtheria, Tetanus and Pertussis Vaccine Market
- In July 2024, BioNet Europe, the French branch of BioNet, announced the submission of a centralized Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) for its monovalent Recombinant Pertussis Vaccine (RPV). This standalone vaccine is designed for individuals who need a pertussis-only booster shot and are current with their diphtheria and tetanus vaccinations.
- In October 2023, Adimmune Corporation announced the successful extension of the marketing authorization for its tetanus vaccine. The company is set to begin the steady supply of at least one million doses annually for the domestic market in Taiwan starting as early as November 2023. This tetanus vaccine helps prevent infections caused by Clostridium tetani bacteria, which can lead to neurological conditions such as muscle stiffness and convulsions.
- In April 2022, the Research Foundation for Microbial Diseases of Osaka University and Mitsubishi Tanabe Pharma Corporation announced that BIKEN had submitted an application for marketing authorization to the Ministry of Health, Labour and Welfare for their combination vaccine (BIKEN Clinical Trial Code: BK1310, MTPC Clinical Trial Code: MT-2355). This vaccine, developed jointly by the two organizations, is intended for the prevention of pertussis, diphtheria, tetanus, acute poliomyelitis (polio), and Haemophilus influenzae type b (Hib).
- In February 2021, Dynavax Technologies Corporation and Serum Institute of India (SIIPL) jointly announced that the first participant had been dosed in a Phase 1 clinical trial for a tetanus, diphtheria, and acellular pertussis (Tdap) booster vaccine candidate adjuvanted with CpG 1018. The two companies are collaborating to develop this adjuvanted Tdap vaccine to overcome the limitations of currently available acellular pertussis vaccines.
- In June 2021, VAXELIS, a hexavalent (six-in-one) combination vaccine developed through a U.S.-based partnership between Merck and Sanofi Pasteur, the global vaccines division of Sanofi, became available in the U.S. VAXELIS is the first and only vaccine in the U.S. to combine Diphtheria and Tetanus Toxoids, Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate, and Hepatitis B.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.