Global Drug Safety Solutions And Pharmacovigilance Market
Market Size in USD Billion
CAGR :
% 
 USD
9.01 Billion
USD
16.08 Billion
2024
2032
USD
9.01 Billion
USD
16.08 Billion
2024
2032
| 2025 –2032 | |
| USD 9.01 Billion | |
| USD 16.08 Billion | |
|
|
|
|
Drug Safety Solutions and Pharmacovigilance Market Size
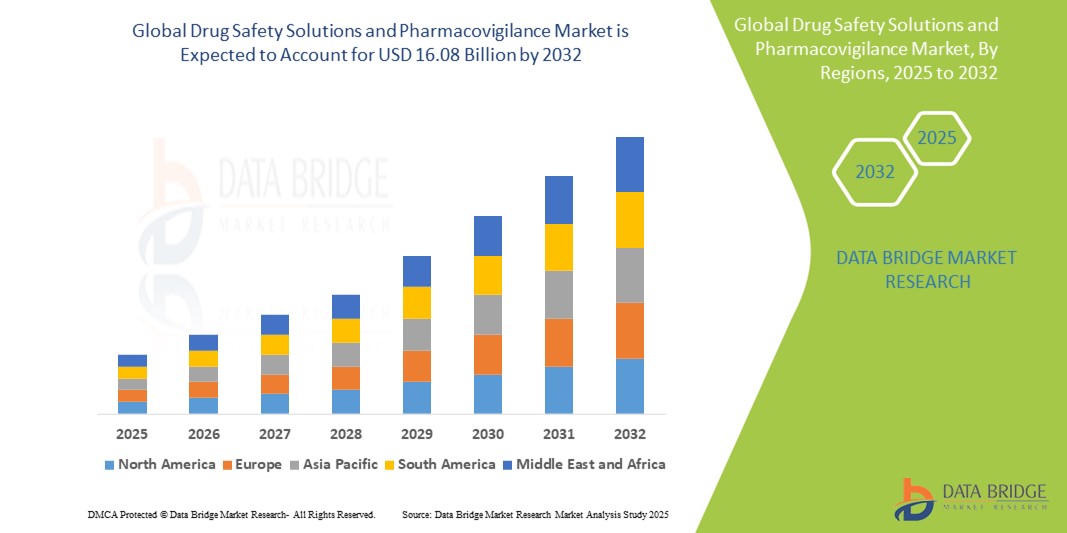
- The global drug safety solutions and pharmacovigilance market size was valued at USD 9.01 billion in 2024 and is expected to reach USD 16.08 billion by 2032, at a CAGR of 7.50% during the forecast period
- The market growth is largely fuelled by the increasing complexity of drug development processes and the surge in clinical trials worldwide, necessitating robust systems for monitoring and managing adverse drug reactions and patient safety data. This has led to greater reliance on advanced drug safety solutions and pharmacovigilance technologies across pharmaceutical and biotech industries
- Furthermore, the rising regulatory pressure from global health authorities and growing public awareness regarding medication risks are driving demand for secure, automated, and compliant safety monitoring systems. These converging factors are accelerating the uptake of drug safety solutions and pharmacovigilance platforms, thereby significantly boosting the industry's growth
Drug Safety Solutions and Pharmacovigilance Market Analysis
- Drug safety solutions and pharmacovigilance systems, providing end-to-end monitoring of adverse drug reactions and safety data throughout a product’s lifecycle, are becoming increasingly vital in both clinical and post-marketing phases due to heightened regulatory scrutiny and the need for real-time risk assessment. These systems are critical for ensuring patient safety, regulatory compliance, and effective pharmacological surveillance
- The escalating demand for drug safety solutions and pharmacovigilance is primarily fueled by the surge in global clinical trials, rising incidence of adverse drug reactions (ADRs), and an increasing emphasis on data integration across pharmacovigilance platforms. The adoption of AI-driven tools, cloud-based reporting systems, and automation technologies has further enhanced the efficiency and accuracy of drug safety operations
- North America dominates the drug safety solutions and pharmacovigilance market with the largest revenue share of 41.3% in 2024, characterized by stringent FDA regulations, a robust pharmaceutical R&D ecosystem, and early adoption of advanced safety monitoring technologies
- Asia-Pacific is expected to be the fastest growing region in the drug safety solutions and pharmacovigilance market during the forecast period, registering a CAGR of 10.4% driven by the rapid expansion of pharmaceutical manufacturing, increasing clinical research activity, rising healthcare expenditures, and government initiatives to align with global drug safety standards
- The software segment dominates the drug safety solutions and pharmacovigilance market with a market share of 47.6% in 2024, propelled by the growing need for centralized databases, real-time monitoring dashboards, and seamless integration with EHR systems. The demand for scalable, cloud-enabled, and AI-powered software platforms continues to rise as companies seek to improve compliance and operational efficiency in safety reporting
Report Scope and Drug Safety Solutions and Pharmacovigilance Market Segmentation
|
Attributes |
Drug Safety Solutions and Pharmacovigilance Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Drug Safety Solutions and Pharmacovigilance Market Trends
“Enhanced Convenience Through Automation and System Integration”
- A significant and accelerating trend in the global drug safety solutions and pharmacovigilance market is the increasing integration of automated platforms with regulatory and clinical data systems. This integration is streamlining adverse event detection, case processing, and regulatory submissions across the pharmaceutical value chain
- For instance, several leading pharmacovigilance software providers now offer centralized platforms that integrate with clinical trial databases, electronic health records (EHRs), and regulatory systems such as EudraVigilance and the FDA’s FAERS, ensuring real-time reporting and compliance
- Automation is enabling features such as intelligent case triaging, duplicate detection, and narrative generation, significantly reducing manual workload and enhancing the accuracy of safety data. Companies are increasingly deploying robotic process automation (RPA) for routine data entry and reconciliation
- The seamless integration of drug safety systems with broader clinical and commercial platforms facilitates a unified safety monitoring environment. Through a single interface, stakeholders can track product safety, signal detection, and benefit-risk assessments across multiple markets and product lines
- This trend toward more intelligent, streamlined, and interconnected pharmacovigilance operations is reshaping regulatory strategies and lifecycle drug safety management. Organizations are prioritizing platforms that support end-to-end pharmacovigilance workflows and enable global compliance
- The demand for drug safety solutions that offer scalable automation, real-time integration, and advanced data analytics is rising rapidly across pharmaceutical, biotech, and CRO sectors, as companies aim to enhance patient safety while optimizing operational efficiency
Drug Safety Solutions and Pharmacovigilance Market Dynamics
Driver
“Growing Need Due to Rising Drug Complexity and Regulatory Pressures”
- The increasing complexity of pharmaceutical products, including biologics, gene therapies, and personalized medicines, has amplified the demand for robust pharmacovigilance systems to monitor drug safety throughout the lifecycle
- For instance, the global shift toward accelerated drug approvals and emergency use authorizations has underscored the importance of real-time safety surveillance to identify and manage adverse drug reactions (ADRs) swiftly
- Regulatory bodies such as the FDA (U.S.), EMA (Europe), and CDSCO (India) are mandating stringent post-marketing surveillance and risk management plans, compelling pharma companies to invest heavily in advanced drug safety solutions
- Moreover, the increasing volume of global clinical trials and the involvement of diverse patient populations necessitate high-quality data integration and adverse event tracking across multiple geographies and regulatory frameworks
- Cloud-based drug safety platforms and AI-driven signal detection tools are being adopted at scale to improve accuracy and efficiency, enabling real-time data analysis and early identification of potential drug risks
- As a result, pharmaceutical and biotech companies are increasingly outsourcing pharmacovigilance functions to specialized vendors offering cost-effective, scalable, and compliant drug safety services, thus accelerating market growth
Restraint/Challenge
“Data Privacy Concerns and Limited Skilled Workforce”
- Despite the growing demand for pharmacovigilance solutions, data privacy and compliance concerns pose significant restraints, especially in regions with evolving data protection laws such as GDPR (Europe), HIPAA (U.S.), and India’s DPDP Act
- For instance, cross-border data transfers and the handling of patient health information in outsourced drug safety operations require rigorous safeguards and legal clarity, often creating operational complexity and delays
- Moreover, the shortage of skilled pharmacovigilance professionals—particularly those with expertise in regulatory writing, safety database management, and global compliance—continues to challenge market scalability
- Small and mid-sized pharmaceutical companies often struggle with the financial and infrastructural burden of maintaining in-house PV teams, leading to fragmented or inconsistent drug safety practices
- Further complicating the landscape are language barriers, inconsistent adverse event reporting standards across countries, and integration issues with legacy systems, which hinder real-time safety monitoring and global data harmonization
- Addressing these challenges requires enhanced industry training programs, stronger regulatory collaboration, and the implementation of secure, interoperable platforms to ensure both compliance and effective pharmacovigilance practices worldwide
Drug Safety Solutions and Pharmacovigilance Market Scope
The drug safety solutions and pharmacovigilance market is segmented on the basis of type, product, functionality, delivery, end users, and distribution channel.
• By Type
On the basis of type, the drug safety solutions and pharmacovigilance market is segmented into software and services. The services segment held the largest revenue share of 47.6% in 2024, driven by rising outsourcing trends among pharmaceutical companies for safety monitoring and regulatory compliance. These services are often tailored and scalable, supporting both established pharma firms and emerging biotechs.
The software segment is anticipated to witness the fastest CAGR of 11.3% from 2025 to 2032, owing to increased adoption of automation tools, machine learning-based adverse event detection, and cloud-based pharmacovigilance platforms.
• By Product
On the basis of product, the drug safety solutions and pharmacovigilance market is segmented into standard form and customized form. The standard form segment dominated the market with a revenue share of 63.1% in 2024, as many end-users prefer cost-effective, ready-to-implement solutions for basic regulatory reporting and safety analysis.
The customized form segment is expected to grow at the fastest CAGR of 10.1% from 2025 to 2032, driven by rising demand for tailored platforms that align with company-specific compliance workflows, therapeutic areas, and global reporting standards.
• By Functionality
On the basis of functionality, the drug safety solutions and pharmacovigilance market is segmented into adverse event reporting software, drug safety audits software, and issue tracking software. The adverse event reporting software segment held the largest revenue share of 54.6% in 2024, as it serves as the backbone of all pharmacovigilance processes and is critical for meeting global health authority requirements.
The issue tracking software segment is projected to register the fastest CAGR of 10.9% during the forecast period, as companies seek real-time issue resolution tools to manage safety case lifecycle complexities and enhance signal detection accuracy.
• By Delivery
On the basis of delivery, the drug safety solutions and pharmacovigilance market is segmented into on-premise delivery mode and on-demand/cloud-based (SaaS) delivery mode. The on-demand/cloud-based delivery mode leads with a market share of 60.3% in 2024, due to its scalability, lower infrastructure costs, and ease of real-time access across global pharmacovigilance teams.
The on-premise delivery segment, though declining in adoption, still holds relevance for highly regulated or security-sensitive operations.
• By End Users
On the basis of end users, the drug safety solutions and pharmacovigilance market is segmented into biotechnology and pharmaceuticals, contract research organizations (CROs), hospitals, KPOs/BPOs, and healthcare providers. The biotechnology and pharmaceutical companies segment dominated with a revenue share of 48.9% in 2024, as these organizations are directly responsible for drug safety compliance, post-marketing surveillance, and regulatory submissions.
The CROs segment is expected to witness the fastest CAGR of 11.7% from 2025 to 2032, driven by increased outsourcing of pharmacovigilance functions for cost efficiency and expertise access.
• By Distribution Channel
On the basis of distribution channel, the drug safety solutions and pharmacovigilance market is segmented into direct sales and retail sales. The direct sales segment accounted for the largest share of 71.6% in 2024, reflecting the complex, high-value nature of pharmacovigilance solution deals that require customized contracts and support.
The retail sales segment is likely to grow at a moderate pace, driven by off-the-shelf software purchases by smaller firms and individual healthcare providers.
Drug Safety Solutions and Pharmacovigilance Market Regional Analysis
- North America dominates the drug safety solutions and pharmacovigilance market with the largest revenue share of 41.3% in 2024, driven by stringent regulatory requirements, the presence of major pharmaceutical companies, and increased investment in drug safety technologies
- The region’s stakeholders highly value advanced pharmacovigilance software and services that ensure compliance with FDA and Health Canada regulations, enabling efficient adverse event reporting and real-time safety monitoring
- This widespread adoption is further supported by high technological awareness, growing demand for cloud-based delivery models, and a strong focus on patient safety, establishing the region as a key hub for Drug Safety Solutions and Pharmacovigilance across pharmaceutical, biotech, and healthcare sectors
U.S. Drug Safety Solutions and Pharmacovigilance Market Insight
The U.S. drug safety solutions and pharmacovigilance market held the largest revenue share of 78% in North America in 2024, driven by the presence of leading pharmaceutical companies and stringent regulatory frameworks such as the FDA. The growing focus on patient safety, increasing clinical trials, and advancements in cloud-based pharmacovigilance software are fueling market growth. Moreover, rising adoption of AI-powered drug safety tools and robust healthcare infrastructure contribute significantly to the expansion of this market.
Europe Drug Safety Solutions and Pharmacovigilance Market Insight
The Europe drug safety solutions and pharmacovigilance market is expected to grow at a strong CAGR from 2025 to 2032, supported by strict regulatory mandates such as the EU Pharmacovigilance Directive. Increasing healthcare expenditure, expanding clinical research activities, and growing awareness of drug safety practices are encouraging adoption across the region. Germany, France, and the U.K. are notable contributors, with rising demand from pharmaceutical companies and contract research organizations.
U.K. Drug Safety Solutions and Pharmacovigilance Market Insight
The U.K. drug safety solutions and pharmacovigilance market is forecasted to grow at a significant CAGR during the forecast period, driven by the nation’s well-established life sciences sector and increasing government initiatives to enhance drug safety monitoring. The emphasis on improving healthcare data analytics and expanding pharmacovigilance outsourcing to Contract Research Organizations (CROs) further stimulate market growth.
Germany Drug Safety Solutions and Pharmacovigilance Market Insight
The Germany drug safety solutions and pharmacovigilance makret is anticipated to expand at a noteworthy CAGR during the forecast period, fueled by increasing investments in digital health technologies and rising adoption of automated adverse event reporting systems. Strong regulatory support combined with advanced healthcare infrastructure accelerates the demand for integrated pharmacovigilance platforms in both pharmaceutical and biotech industries.
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Insight
The Asia-Pacific drug safety solutions and pharmacovigilance market is projected to register the highest CAGR of 10.4% between 2025 and 2032, driven by rapid urbanization, government healthcare reforms, and growing clinical research activities in countries such as China, India, Japan, and South Korea. Increasing pharmaceutical manufacturing, rising awareness about drug safety, and the growing adoption of cloud-based solutions contribute to robust market expansion.
Japan Drug Safety Solutions and Pharmacovigilance Market Insight
The Japan drug safety solutions and pharmacovigilance market growth is steady, expected to grow at a CAGR of 14%, driven by its aging population, advanced healthcare ecosystem, and increasing implementation of AI-based drug safety monitoring systems. Integration of pharmacovigilance with electronic health records (EHR) and strong regulatory oversight are key factors supporting expansion.
China Drug Safety Solutions and Pharmacovigilance Market Insight
The China drug safety solutions and pharmacovigilance accounts for the largest revenue share in the Asia-Pacific region at 35% in 2024, fueled by its rapidly growing pharmaceutical sector, large-scale clinical trials, and government emphasis on improving drug safety standards. The rise of smart healthcare infrastructure and domestic software development firms also propel market adoption across the country.
Drug Safety Solutions and Pharmacovigilance Market Share
The drug safety solutions and pharmacovigilance industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc (U.S.)
- C3i (U.S.)
- Worldwide Clinical Trials (U.S.)
- Clario (U.S.)
- United Biosource LLC (U.S.)
- Ennov (Hong Kong)
- AB Cube S.A.S. (France)
- Labcorp (U.S.)
- Accenture (Ireland)
- ICON plc (U.S.)
- ERGOMED Group (U.K.)
- IQVIA (U.S.)
- Genpact (U.S.)
- Cognizant (U.S.)
- Parexel International (MA) Corporation (U.S.)
- ArisGlobal (U.S.)
Latest Developments in Global Drug Safety Solutions and Pharmacovigilance Market
- In May 2025, IQVIA, a global leader in clinical research and data analytics, announced the launch of its enhanced pharmacovigilance platform designed to streamline adverse event reporting and improve drug safety monitoring worldwide. The platform leverages advanced analytics and real-time data integration to provide faster insights, enabling healthcare providers and pharmaceutical companies to make informed safety decisions. This innovation underscores IQVIA’s commitment to advancing drug safety and regulatory compliance
- In April 2025, Cognizant expanded its Drug Safety Solutions portfolio by integrating AI-driven automation tools to enhance case processing efficiency and accuracy. The company reported significant reductions in manual data entry errors and faster turnaround times for safety reports. This strategic development reflects Cognizant’s focus on digital transformation in pharmacovigilance services
- In March 2025, Parexel International Corporation partnered with a leading biotech firm to deploy its cloud-based pharmacovigilance system across multiple clinical trial sites globally. This initiative aims to unify safety data collection and reporting, ensuring consistent regulatory compliance and improved patient safety across trials. The collaboration highlights Parexel’s role in facilitating innovative drug safety management solutions
- In February 2025, ArisGlobal launched its latest version of the LifeSphere Drug Safety platform, featuring enhanced risk management and signal detection capabilities powered by machine learning. This update offers pharmaceutical companies greater precision in monitoring drug safety signals and supports faster regulatory submissions, reinforcing ArisGlobal’s position as a pioneer in pharmacovigilance technology
- In January 2025, Labcorp Drug Development announced the integration of real-world evidence data into its drug safety monitoring services to better assess post-market safety profiles. The company emphasized that this holistic approach enhances pharmacovigilance outcomes by combining clinical trial data with patient data from diverse healthcare settings, promoting safer drug use globally
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.