Global Drugs Of Abuse Doa Testing Market
Market Size in USD Million
CAGR :
% 
 USD
6,253.25 Million
USD
13,650.06 Million
2022
2030
USD
6,253.25 Million
USD
13,650.06 Million
2022
2030
| 2023 –2030 | |
| USD 6,253.25 Million | |
| USD 13,650.06 Million | |
|
|
|
|
Drugs of Abuse (DOA) Testing Market Analysis and Size
According to the United Nations Office on Drugs and Crime's World Drug Report 2017, the annual prevalence of all illicit drug use is 5.3%, with 255 million users. This will necessitate the need for DOA testing, propelling the market forward. The government's initiatives to raise awareness about DOA and increase organizational compliance for DOA testing will contribute to the overall market's growth. The high demand for products with increased specificity and sensitivity to designer drugs will also contribute to the expansion of the Drug of Abuse (DOA) testing market.
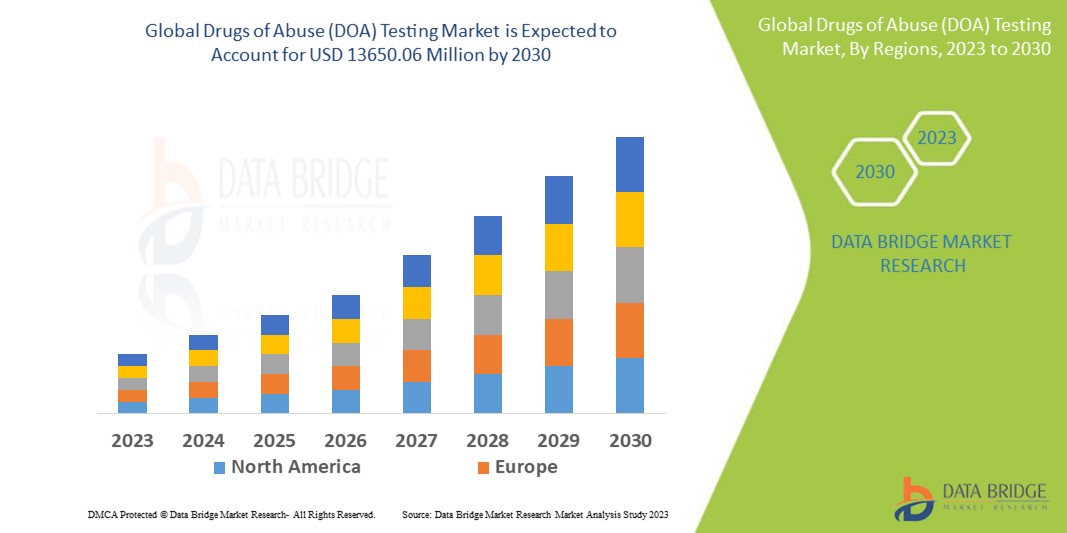
Data Bridge Market Research analyses that the drugs of abuse (DOA) testing market, which is USD 6253.25 million in 2022, is expected to reach USD 13650.06 million by 2030, at a CAGR of 10.25% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Drugs of Abuse (DOA) Testing Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Consumables, Analyzers, Equipment), Sample Type (Urine, Saliva, Hair, Others), Application (Pain Management, Criminal Justice, Workplace Screening), End Users (Hospitals, Laboratories, Workplace, At Home, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Takeda Pharmaceutical Company Limited (Japan), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (U.S.), Medtronic (Ireland), Abbott (U.S.), BD (U.S.), Johnson & Johnson Services, Inc., GSK Plc. (U.K.), Bayer AG (Germany), Zimmer Biomet (U.S.), Stryker Corporation (U.S.), Homology Medicines, Inc (U.S.), Novartis AG (Switzerland), Pfizer Inc.(U.S.), JCR Pharmaceuticals Co., Ltd. (Japan), Sangamo Therapeutics (U.S.), AVROBIO, Inc (U.S.), REGENXBIO Inc (U.S), CANbridge Life Sciences Ltd. (Taiwan), Denali Therapeutics (U.S.), and Jasper Therapeutics, Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Drug of Abuse testing is a clinical screening procedure used to detect one or more illegal substances such as a drug, chemical, or plant product the patient is addicted to. This clinical screening method uses the patient's urine, saliva, blood, hair, or sweat.
Drugs of Abuse (DOA) Testing Market Dynamics
Drivers
- Increase in consumption and trade of illicit drugs
The increased production, consumption, and trade of new and illicit drugs will result in a high demand for DoA testing, driving industry growth. According to the United Nations Office on Drugs and Crime's World Drug Report 2017, the annual prevalence of all illicit drug use is 5.3%, with 255 million users in 2015. This will necessitate the need for DOA testing, propelling the market forward. The government's initiatives to raise awareness about DOA and increase organizational compliance for DOA testing will contribute to the overall market's growth. The high demand for products with increased specificity and sensitivity to designer drugs will also contribute to the industry's growth.
- Technological advancements and product portfolio expansion
To maintain their competency and market share, leading players in the DoA testing market are expanding their product portfolio through product additions and software updates with new substances for DoA testing. For instance, Shimadzu Corporation, released its Smart Forensic Database Ver. 2 in February 2018, with features for analysing forensic toxicological substances involved in DoA in biological samples using Gas Chromatograph-Mass Spectrometry. Similarly, Thermo Fisher Scientific Inc. updated its library for the Thermo Scientific TruNarc handheld narcotics analyzer in November 2017. The update added 45 new substances to the handheld narcotics analyzer, including 14 new forms of fentanyl, and it can now detect Carfentanil.
Opportunities
- Rising ageing population
According to data published by the Organization for Economic Cooperation and Development (OECD) in 2020, nearly one-third of adults in the European Union aged 15-64, or approximately 97 million people, have used illicit drugs at some point in their lives, with men reporting drug use more frequently than women. To maintain their competency and market shares, leading players in the drug abuse testing market are expanding their product portfolios by adding new products and updating software with new substances for drug abuse testing. These are the certain reasons for propelling the market growth.
Restraints/Challenges
- Inability of testing products to detect small amounts of special drugs
The inability of these testing products to detect small amounts of special drugs is expected to hamper the market growth. In the forecast period of 2023-2030, the transformation of laws to legalize the use of recreational drugs/illicit drugs is expected to challenge the drugs of abuse (DOA) testing market.
This drugs of abuse (DOA) testing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the drugs of abuse (DOA) testing market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2020, WellCare Health Plans introduced new clinical coverage guidelines and claims payment policy for urine drug testing, including definitive urine drug testing as medically necessary as part of a routine monitoring programme for individuals receiving treatment for chronic pain with prescription opioid or other potentially abused medications, or those undergoing treatment for, or monitoring for relapse of, opioid addiction or substance use disorder.
- In 2022, the Delaware Division of Public Health began including fentanyl strips in Narcan kits for public distribution. The initiative was part of a harm-reduction strategy aimed at preventing accidental fentanyl overdoses.
Global Drugs of Abuse (DOA) Testing Market Scope
The drugs of abuse (DOA) testing market is segmented on the basis of product, sample type, application and end users. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Consumables
- Rapid test kits
- Assay kits
- Reagents
- Others
- Blood
- Breath
- Analyzers
- Equipment
- Immunoassay analyzers
- Chromatography instruments
- Breath analyzers
Sample Type
- Urine
- Saliva
- Hair
- Others
Application
- Pain Management
- Criminal Justice
- Workplace Screening
End Users
- Hospitals
- Laboratories
- Workplace
- At Home
- Others
Drugs of Abuse (DOA) Testing Market Regional Analysis/Insights
The drugs of abuse (DOA) testing market is analyzed and market size insights and trends are provided by country, product, sample type, application and end users as referenced above.
The countries covered in the drugs of abuse (DOA) testing market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the drugs of abuse (DOA) testing market due to the region's high availability of illegal substances, rising drug trafficking, and more workplace monitoring for drug use.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 due to the region's rising use of illegal substances and rise in organizational adherence to workplace drug testing.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The drugs of abuse (DOA) testing market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for drugs of abuse (DOA) testing market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the drugs of abuse (DOA) testing market. The data is available for historic period 2011-2021.
Competitive Landscape and Drugs of Abuse (DOA) Testing Market Share Analysis
The drugs of abuse (DOA) testing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to drugs of abuse (DOA) testing market.
Some of the major players operating in the drugs of abuse (DOA) testing market are:
- Takeda Pharmaceutical Company Limited (Japan)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Medtronic (Ireland)
- Abbott (U.S.)
- BD (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- GSK Plc. (U.K.)
- Bayer AG (Germany)
- Zimmer Biomet (U.S.)
- Stryker Corporation (U.S.)
- Homology Medicines, Inc (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- Sangamo Therapeutics (U.S.)
- AVROBIO, Inc (U.S.)
- REGENXBIO Inc (U.S)
- CANbridge Life Sciences Ltd. (Taiwan)
- Denali Therapeutics (U.S.)
- Jasper Therapeutics, Inc. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL XX SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
8.7 OTHERS (PRE-CLINICAL AND RESEARCH)
9 MARKET OVERVIEW
9.1 DRIVERS
9.2 RESTRAINTS
9.3 OPPORTUNITIES
9.4 CHALLENGES
10 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY PRODUCT
10.1 OVERVIEW
10.2 EQUIPMENTS
10.2.1 BY TYPE
10.2.1.1. HANDHELD
10.2.1.2. FIXED
10.2.1.3. MOBILES
10.2.2 BY MODALITY
10.2.2.1. AUTOMATED
10.2.2.2. MANUAL
10.2.3 BY PRODUCT
10.2.3.1. ANALYSERS
10.2.3.1.1. IMMUNOASSAY ANALYZERS
10.2.3.1.2. BREATH ANALYZERS
10.2.3.2. CHROMATOGRAPHIC DEVICES
10.2.3.3. RAPID TESTING DEVICES
10.2.3.3.1. URINE TESTING DEVICES
10.2.3.3.2. ORAL FLUID TESTING DEVICES
10.2.3.4. OTHERS
10.3 CONSUMABLES
10.3.1 RAPID TEST KITS
10.3.1.1. STRIPS
10.3.1.1.1. MARKET VALUE (USD MILLION)
10.3.1.1.2. MARKET VOLUME (UNITS)
10.3.1.1.3. AVERAGE SELLING PRICE (USD)
10.3.1.2. CASSETTES
10.3.1.2.1. MARKET VALUE (USD MILLION)
10.3.1.2.2. MARKET VOLUME (UNITS)
10.3.1.2.3. AVERAGE SELLING PRICE (USD)
10.3.1.3. CUPS
10.3.1.3.1. MARKET VALUE (USD MILLION)
10.3.1.3.2. MARKET VOLUME (UNITS)
10.3.1.3.3. AVERAGE SELLING PRICE (USD)
10.3.1.4. OTHERS
10.3.2 ASSAY KITS
10.3.2.1. ENZYME IMMUNOASSAY (EIA) KITS
10.3.2.1.1. MARKET VALUE (USD MILLION)
10.3.2.1.2. MARKET VOLUME (UNITS)
10.3.2.1.3. AVERAGE SELLING PRICE (USD)
10.3.2.2. FLUORESCENCE IMMUNOASSAY (FIA) KITS
10.3.2.2.1. MARKET VALUE (USD MILLION)
10.3.2.2.2. MARKET VOLUME (UNITS)
10.3.2.2.3. AVERAGE SELLING PRICE (USD)
10.3.2.3. CHEMILUMINESCENCE IMMUNOASSAY (CLIA) KITS
10.3.2.3.1. MARKET VALUE (USD MILLION)
10.3.2.3.2. MARKET VOLUME (UNITS)
10.3.2.3.3. AVERAGE SELLING PRICE (USD)
10.3.2.4. RAPID LATERAL FLOW IMMUNOASSAY (LFIA) KITS
10.3.2.4.1. MARKET VALUE (USD MILLION)
10.3.2.4.2. MARKET VOLUME (UNITS)
10.3.2.4.3. AVERAGE SELLING PRICE (USD)
10.3.2.5. RADIOIMMUNOASSAY (RIA) KITS
10.3.2.5.1. MARKET VALUE (USD MILLION)
10.3.2.5.2. MARKET VOLUME (UNITS)
10.3.2.5.3. AVERAGE SELLING PRICE (USD)
10.4 REAGENTS
10.5 OTHERS
11 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY SAMPLE TYPE
11.1 OVERVIEW
11.2 URINE
11.3 SALIVA
11.4 HAIR
11.5 BREATH
11.6 OTHERS
12 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY MODE
12.1 OVERVIEW
12.2 POINT OF CARE TESTING
12.3 LABORATORY BASED TESTING
13 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY APPLICATION
13.1 OVERVIEW
13.2 MEDICAL SCREENING
13.3 PAIN MANAGEMENT
13.4 LAW ENFORCEMENT AND CRIMINAL JUSTICE
13.5 WORKPLACE SCREENING
13.6 SUBSTANCE ABUSE TREATMENT AND REHABILITATION
13.7 DRUG SCREENING IN SCHOOLS AND EDUCATIONAL INSTITUTIONS
13.8 PARENTAL OR HOME DRUG TESTING
13.9 SPORTS AND ATHLETICS TESTINGS
13.1 OTHERS
14 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY DRUG TYPE
14.1 OVERVIEW
14.2 ALCOHOL
14.3 COCAINE
14.4 MARIJUANA/ CANNABIS
14.5 LYSERGIC ACID DIETHYLAMIDE
14.6 OPIOIDS
14.7 OTHERS
15 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 LABORATORIES
15.4 WORKPLACE
15.5 AT HOME
15.6 OTHERS
16 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDERS
16.3 RETAIL SALES
16.4 ONLINE
16.5 OTHERS
17 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , SWOT AND DBMR ANALYSIS
18 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: GLOBAL
18.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.3 COMPANY SHARE ANALYSIS: EUROPE
18.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , BY REGION
19.1 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
19.2 NORTH AMERICA
19.2.1 U.S.
19.2.2 CANADA
19.2.3 MEXICO
19.3 EUROPE
19.3.1 GERMANY
19.3.2 U.K.
19.3.3 ITALY
19.3.4 FRANCE
19.3.5 SPAIN
19.3.6 RUSSIA
19.3.7 SWITZERLAND
19.3.8 TURKEY
19.3.9 BELGIUM
19.3.10 NETHERLANDS
19.3.11 DENMARK
19.3.12 SWEDEN
19.3.13 POLAND
19.3.14 NORWAY
19.3.15 FINLAND
19.3.16 REST OF EUROPE
19.4 ASIA-PACIFIC
19.4.1 JAPAN
19.4.2 CHINA
19.4.3 SOUTH KOREA
19.4.4 INDIA
19.4.5 SINGAPORE
19.4.6 THAILAND
19.4.7 INDONESIA
19.4.8 MALAYSIA
19.4.9 PHILIPPINES
19.4.10 AUSTRALIA
19.4.11 NEW ZEALAND
19.4.12 VIETNAM
19.4.13 TAIWAN
19.4.14 REST OF ASIA-PACIFIC
19.5 SOUTH AMERICA
19.5.1 BRAZIL
19.5.2 ARGENTINA
19.5.3 REST OF SOUTH AMERICA
19.6 MIDDLE EAST AND AFRICA
19.6.1 SOUTH AFRICA
19.6.2 EGYPT
19.6.3 BAHRAIN
19.6.4 UNITED ARAB EMIRATES
19.6.5 KUWAIT
19.6.6 OMAN
19.6.7 QATAR
19.6.8 SAUDI ARABIA
19.6.9 REST OF MEA
19.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
20 GLOBAL DRUGS OF ABUSE (DOA) TESTING MARKET , COMPANY PROFILE
20.1 EPPENDORF SE
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 THERMO FISHER SCIENTIFIC INC.
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 BECKMAN COULTER, INC.
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 BMG LABTECH
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 DRÄGERWERK AG & CO. KGAA
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 LIFELOC TECHNOLOGIES, INC
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 INTOXIMETERS
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 CMI INC.
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 ABBOTT
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 ALERE.
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 BIO-RAD LABORATORIES, INC.
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 SIEMENS HEALTHCARE PRIVATE LIMITED
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 F. HOFFMANN-LA ROCHE LTD
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 AK GLOBALTECH CORPORATION
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 C4 DEVELOPMENT LTD
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 BACTRACK
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 LION LABORATORIES LIMITED
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
20.18 SECURETEC
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPMENTS
20.19 CONTRALCO
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPMENTS
20.2 ALCOHOL COUNTERMEASURE SYSTEMS CORP
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPMENTS
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.