Global Electronic Trial Master File Etmf Systems Market
Market Size in USD Billion
CAGR :
% 
 USD
1.84 Billion
USD
4.85 Billion
2024
2032
USD
1.84 Billion
USD
4.85 Billion
2024
2032
| 2025 –2032 | |
| USD 1.84 Billion | |
| USD 4.85 Billion | |
|
|
|
|
Electronic Trial Master File (eTMF) Systems Market Size
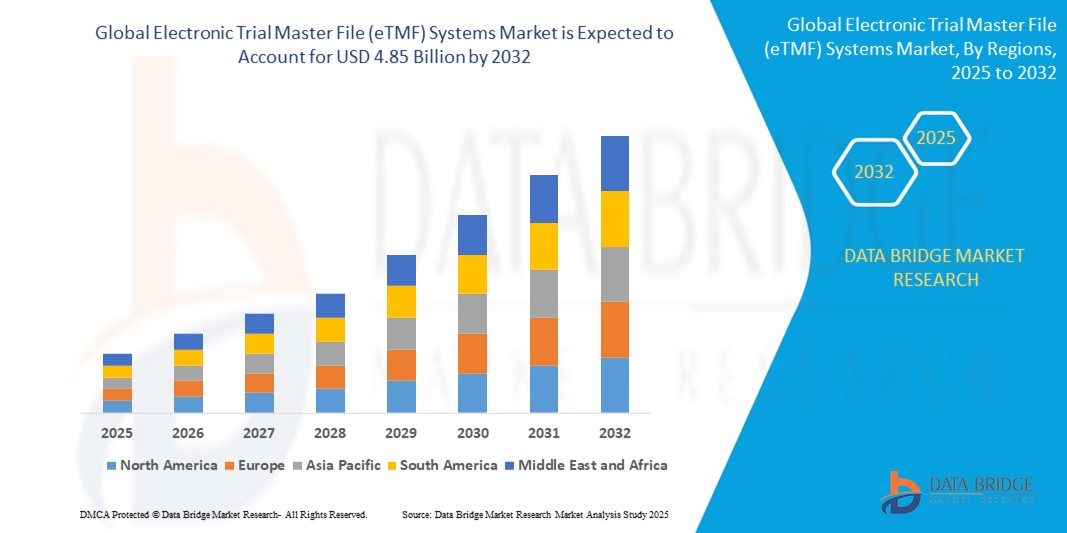
- The global electronic trial master file (eTMF) systems market size was valued at USD 1.84 billion in 2024 and is expected to reach USD 4.85 billion by 2032, at a CAGR of 12.90% during the forecast period
- The market growth is largely fuelled by the increasing demand for centralized clinical trial data management, regulatory compliance, and the growing adoption of digital platforms in clinical research across pharmaceutical and biotechnology sectors
- The rising number of clinical trials globally, especially in emerging markets, and the need for real-time access to trial documents are further accelerating the adoption of electronic trial master file (eTMF) systems across sponsors and contract research organizations
Electronic Trial Master File (eTMF) Systems Market Analysis
- The electronic trial master file systems market is witnessing steady growth as pharmaceutical and biotechnology companies increasingly adopt digital solutions for clinical document management
- Rising clinical trial activities and the need for efficient regulatory compliance processes are encouraging organizations to shift from paper-based systems to cloud-based platforms
- North America dominated the electronic trial master file (eTMF) systems market with the largest revenue share of 49.5% in 2024, driven by a high concentration of pharmaceutical and biotechnology companies, substantial R&D investments, a well-developed healthcare IT infrastructure, and a growing number of complex clinical trials requiring efficient documentation and compliance
- Asia-Pacific region is expected to witness the highest growth rate in the global electronic trial master file (eTMF) systems market, driven by increasing urbanization, rising disposable incomes, technological advancements in countries such as China, Japan, and India, and a growing inclination towards smart homes supported by government digitalization initiatives
- The Services segment held the largest market revenue share in 2024, due to essential offerings throughout the system lifecycle, including implementation, training, technical support, and data migration
Report Scope and Electronic Trial Master File (eTMF) Systems Market Segmentation
|
Attributes |
Electronic Trial Master File (eTMF) Systems Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand. |
Electronic Trial Master File (eTMF) Systems Market Trends
“Integration of Artificial Intelligence for Workflow Automation”
- Artificial intelligence is increasingly being integrated into electronic trial master file systems to automate repetitive tasks such as document classification and metadata tagging
- This trend improves efficiency and reduces manual errors, allowing clinical research professionals to focus more on strategic and compliance-related tasks
- For instance, AI-powered platforms can auto-index clinical trial documents and flag missing files, ensuring greater accuracy and regulatory readiness
- Companies such as Veeva Systems and Phlexglobal are adopting AI capabilities to streamline eTMF processes and provide real-time insights into trial status
- With growing volumes of clinical data, AI integration is becoming essential in enhancing decision-making and speeding up the clinical trial lifecycle
Electronic Trial Master File (eTMF) Systems Market Dynamics
Driver
“Shift Toward Digitization in Clinical Trial Management”
- The shift toward digitization is driving the adoption of electronic trial master file systems as clinical trials grow more complex and require more efficient document management
- These systems provide centralized access to trial documents, allowing for faster retrieval, real-time collaboration, and improved version control across teams
- For instance, large pharmaceutical companies such as Pfizer have adopted digital trial management platforms to streamline documentation and ensure regulatory compliance
- Digital systems help reduce risks associated with manual errors, lost documents, and delayed submissions, ultimately improving trial quality and timelines
- Remote access and secure cloud storage features make these systems essential for organizations aiming to enhance operational efficiency and meet global regulatory standards
Restraint/Challenge
“Data Security and Compliance Concerns”
- Data security and regulatory compliance concerns remain major restraints in the adoption of electronic trial master file systems across the clinical research industry
- Clinical trial data is highly sensitive, and any breach can lead to serious consequences such as trial delays, legal repercussions, and damage to organizational credibility
- For instance, a reported data breach at a European clinical research organization led to regulatory investigations and delayed trial timelines, underscoring the risks involved
- Compliance with evolving international regulations such as the General Data Protection Regulation and Health Insurance Portability and Accountability Act demands constant updates and region-specific protocols
- Small and mid-sized organizations often face challenges in affording the necessary cybersecurity infrastructure and expertise, limiting their ability to fully transition to cloud-based eTMF platforms
Electronic Trial Master File (eTMF) Systems Market Scope
The market is segmented on the basis of component, delivery mode, and end-user.
- By Component
On the basis of component, the eTMF systems market is segmented into services and software. The Services segment held the largest market revenue share in 2024, due to essential offerings throughout the system lifecycle, including implementation, training, technical support, and data migration.
The Software segment is projected to witness the fastest growth rate from 2025 to 2032, as organizations increasingly adopt dedicated eTMF platforms for centralized document management and regulatory compliance.
- By Delivery Mode
On the basis of delivery mode, the eTMF systems market is segmented into cloud-based eTMF and on-premise eTMF. The Cloud-Based eTMF segment held the largest market revenue share in 2024, driven by enhanced accessibility, flexibility, cost-effectiveness, and scalability.
The On-Premise eTMF segment is projected to witness the fastest growth rate from 2025 to 2032, remains relevant for organizations prioritizing full control over data security and infrastructure, often due to specific regulatory or internal IT policies.
- By End-User
On the basis of end-user, the eTMF systems market is segmented into Pharmaceutical and Biotechnology Companies, CROs, and others. Pharmaceutical and Biotechnology Companies segment held the largest market revenue share in 2024, driven by increasingly complex clinical trials, globalization, and stringent regulatory requirements.
Contract Research Organizations (CROs) is projected to witness the fastest growth rate from 2025 to 2032, as they increasingly leverage eTMF systems to manage diverse trials for various sponsors efficiently.
Electronic Trial Master File (eTMF) Systems Market Regional Analysis
- North America dominated the electronic trial master file (eTMF) systems market with the largest revenue share of 49.5% in 2024, driven by a high concentration of pharmaceutical and biotechnology companies, substantial R&D investments, a well-developed healthcare IT infrastructure, and a growing number of complex clinical trials requiring efficient documentation and compliance
- Stringent regulatory environment and emphasis on data integrity, pushing for robust and compliant eTMF solutions to meet FDA and other health authority requirements
- High adoption of advanced cloud-based technologies and digital transformation initiatives within clinical research organizations, further accelerating eTMF system implementation and utilization
U.S. Electronic Trial Master File (eTMF) Systems Market Insight
The U.S. eTMF systems market held the largest revenue share of 81% in 2024 within North America, fueled by stringent regulatory requirements, the increasing complexity and volume of clinical trial data, and a strong emphasis on audit readiness. The robust adoption of cloud-based solutions and government support for healthcare R&D further propel market expansion.
Europe Electronic Trial Master File (eTMF) Systems Market Insight
The Europe market is projected to witness the fastest growth rate from 2025 to 2032, driven by increasing demand for cloud-based solutions, the necessity for improved data sharing and collaboration among stakeholders, and evolving regulatory mandates. The region also benefits from a strong focus on innovation and digital transformation in the life sciences sector.
U.K. Electronic Trial Master File (eTMF) Systems Market Insight
The U.K. eTMF systems market is projected to witness the fastest growth rate from 2025 to 2032, driven by the escalating number of clinical trials, particularly with an increasing emphasis on decentralized and virtual trials. The country's robust pharmaceutical industry and a proactive approach to digital health initiatives contribute to market growth.
Germany Electronic Trial Master File (eTMF) Systems Market Insight
The Germany eTMF systems market is projected to witness the fastest growth rate from 2025 to 2032, fueled by increasing awareness of digital security, strong regulatory compliance needs, and a demand for technologically advanced solutions within its well-developed pharmaceutical sector. Germany's emphasis on innovation and robust industrial base further promotes eTMF adoption.
Asia-Pacific Electronic Trial Master File (eTMF) Systems Market Insight
The Asia-Pacific eTMF systems market is projected to witness the fastest growth rate from 2025 to 2032, driven by increasing urbanization, rising disposable incomes, and significant technological advancements in countries such as China, Japan, and India. The region's growing inclination towards smart clinical operations, supported by government initiatives promoting digitalization, is fostering the adoption of eTMF systems.
Japan Electronic Trial Master File (eTMF) Systems Market Insight
The Japan eTMF systems market is projected to witness the fastest growth rate from 2025 to 2032, due to the country’s high-tech culture, rapid urbanization, and an increasing emphasis on data integrity and regulatory compliance. The market places significant importance on security and efficiency, with adoption driven by the rising number of clinical trials and the integration of eTMF with other digital clinical solutions.
China Electronic Trial Master File (eTMF) Systems Market Insight
The China eTMF systems market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the country's expanding middle class, rapid urbanization, and high rates of technological adoption in healthcare. China stands as a significant hub for clinical research, with strong domestic manufacturers and government support for digital transformation propelling eTMF adoption in residential, commercial, and research settings.
Electronic Trial Master File (eTMF) Systems Market Share
The Electronic Trial Master File (eTMF) Systems industry is primarily led by well-established companies, including:
- IQVIA Inc. (U.S.)
- Labcorp Drug Development (U.S.)
- TransPerfect (U.S.)
- Oracle (U.S.)
- Phlexglobal (U.S.)
- SureClinical Inc. (U.S.)
- Aurea, Inc. (U.S.)
- Veeva Systems (U.S.)
- MasterControl Solutions, Inc. (U.S.)
- Clinevo Technologies (India)
- Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
- Montrium Inc. (U.S.)
- NCGD Inc. (U.S.)
- PharmaVigilance (U.S.)
Latest Developments in Global Electronic Trial Master File (eTMF) Systems Market
- In February 2023, Vial collaborated with Egnyte to integrate the Egnyte eTMF solution into its platform. This aims to streamline document and data processing, ensuring compliance with 21 CFR Part 11 and bolstering audit readiness for life sciences companies, ultimately enhancing efficiency and regulatory adherence in clinical trials
- In September 2022, Montrium launched expert-led TMF services and educational training. This initiative is designed to bolster clinical operations and TMF teams throughout the clinical development process, providing essential support and knowledge to optimize trial management and data integrity in the market
- In June 2022, Anju Software Inc. launched eTMF Master, a new cloud-based eTMF software. This solution facilitates collaboration between sponsors, CROs, and sites for efficient and secure management of clinical trial content while adhering to regulatory standards, thereby accelerating workflows and improving overall compliance in clinical research
- In April 2021, Phlexglobal announced their TMF Quality Review solution. Aimed at pharmaceutical companies, especially global leaders, this offering helps to assess and minimize regulatory risks during mergers and acquisitions, significantly enhancing inspection readiness and ensuring data quality across the market
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMAPANY MARKET SHARE ANALYSIS
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 STANDARDS OF MEASUREMENT
2.2.8 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.9 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHT
5.1 PORTERS FIVE FORCES
5.2 REGULATORY STANDARDS
5.3 TECHNOLOGICAL TRENDS
5.4 PATENT ANALYSIS
5.5 CASE STUDY
5.6 VALUE CHAIN ANALYSIS
5.7 COMPANY COMPARITIVE ANALYSIS
5.8 PRICING ANALYSIS
6 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY OFFERING
6.1 OVERVIEW
6.2 SOFTWARE
6.3 SERVICES
6.3.1 PROFESSIONAL SERVICES
6.3.1.1. TRAINING AND CONSULTING
6.3.1.2. IMPLEMENTATION
6.3.1.3. SUPPORT AND MAINTENANCE
6.3.2 MANAGED SERVICES
7 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY LEVEL OF DOCUMENTS
7.1 OVERVIEW
7.2 STUDY LEVEL DOCUMENTS
7.3 COUNTRY LEVEL DOCUMENTS
7.4 SITE LEVEL DOCUMENTS
8 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY OPERATING SYSTEM
8.1 OVERVIEW
8.2 WINDOWS
8.3 LINUX
8.4 MAC
8.5 MOBILE
8.5.1 ANDROID
8.5.2 IPHONE
8.5.3 IPAD
9 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY DEPLOYMENT MODE
9.1 OVERVIEW
9.2 ON PREMISES
9.3 CLOUD
9.3.1 PUBLIC
9.3.2 PRIVATE
9.3.3 HYBRID
10 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY FUNCTIONALITY
10.1 OVERVIEW
10.2 COMPREHENSIVE SECURITY
10.3 DOCUMENT UPLOADING/CREATION
10.4 AUDITING
10.5 METADATA
10.6 DOCUMENT CLASSIFICATION AND INDEXING
10.7 DOCUMENT STORAGE
10.8 DATA CAPTURING
10.9 SEARCH AND RETRIEVAL
10.1 OTHERS
11 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY PRICING MODEL
11.1 OVERVIEW
11.2 FREE
11.3 SUBSCRIPTION BASED
11.3.1 ANNUAL SUBSCRIPTION
11.3.2 MONTHLY SUBSCRIPTION
12 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY END USER
12.1 OVERVIEW
12.2 BIOTECH & PHARMA COMPANIES
12.2.1 BY OFFERING
12.2.1.1. SOFTWARE
12.2.1.2. SERVICES
12.2.1.2.1. PROFESSIONAL SERVICES
12.2.1.2.1.1 TRAINING AND CONSULTING
12.2.1.2.1.2 IMPLEMENTATION
12.2.1.2.1.3 SUPPORT AND MAINTENANCE
12.2.1.2.2. MANAGED SERVICES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.3.1 BY OFFERING
12.3.1.1. SOFTWARE
12.3.1.2. SERVICES
12.3.1.2.1. PROFESSIONAL SERVICES
12.3.1.2.1.1 TRAINING AND CONSULTING
12.3.1.2.1.2 IMPLEMENTATION
12.3.1.2.1.3 SUPPORT AND MAINTENANCE
12.3.1.2.2. MANAGED SERVICES
12.4 SPONSORS
12.4.1 BY OFFERING
12.4.1.1. SOFTWARE
12.4.1.2. SERVICES
12.4.1.2.1. PROFESSIONAL SERVICES
12.4.1.2.1.1 TRAINING AND CONSULTING
12.4.1.2.1.2 IMPLEMENTATION
12.4.1.2.1.3 SUPPORT AND MAINTENANCE
12.4.1.2.2. MANAGED SERVICES
12.5 OTHERS
13 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY GEOGRAPHY
13.1 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
13.1.1 NORTH AMERICA
13.1.1.1. U.S.
13.1.1.2. CANADA
13.1.1.3. MEXICO
13.1.2 EUROPE
13.1.2.1. GERMANY
13.1.2.2. FRANCE
13.1.2.3. U.K.
13.1.2.4. ITALY
13.1.2.5. SPAIN
13.1.2.6. RUSSIA
13.1.2.7. TURKEY
13.1.2.8. BELGIUM
13.1.2.9. NETHERLANDS
13.1.2.10. NORWAY
13.1.2.11. FINLAND
13.1.2.12. SWITZERLAND
13.1.2.13. DENMARK
13.1.2.14. SWEDEN
13.1.2.15. POLAND
13.1.2.16. REST OF EUROPE
13.1.3 ASIA PACIFIC
13.1.3.1. JAPAN
13.1.3.2. CHINA
13.1.3.3. SOUTH KOREA
13.1.3.4. INDIA
13.1.3.5. AUSTRALIA
13.1.3.6. NEW ZEALAND
13.1.3.7. SINGAPORE
13.1.3.8. THAILAND
13.1.3.9. MALAYSIA
13.1.3.10. INDONESIA
13.1.3.11. PHILIPPINES
13.1.3.12. TAIWAN
13.1.3.13. VIETNAM
13.1.3.14. REST OF ASIA PACIFIC
13.1.4 SOUTH AMERICA
13.1.4.1. BRAZIL
13.1.4.2. ARGENTINA
13.1.4.3. REST OF SOUTH AMERICA
13.1.5 MIDDLE EAST AND AFRICA
13.1.5.1. SOUTH AFRICA
13.1.5.2. EGYPT
13.1.5.3. SAUDI ARABIA
13.1.5.4. U.A.E
13.1.5.5. OMAN
13.1.5.6. BAHRAIN
13.1.5.7. ISRAEL
13.1.5.8. KUWAIT
13.1.5.9. QATAR
13.1.5.10. REST OF MIDDLE EAST AND AFRICA
13.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
14 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET,COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
14.5 MERGERS & ACQUISITIONS
14.6 NEW PRODUCT DEVELOPMENT AND APPROVALS
14.7 EXPANSIONS
14.8 REGULATORY CHANGES
14.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
15 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, SWOT & DBMR ANALYSIS
16 GLOBAL ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, COMPANY PROFILE
16.1 ADLIB
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 GEOGRAPHIC PRESENCE
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 VEEVA MEDTECH(VEEVA SYSTEMS, INC)
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 GEOGRAPHIC PRESENCE
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENT
16.3 TRIAL INTERACTIVE
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 GEOGRAPHIC PRESENCE
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 MEDIDATA ( DASSAULT SYSTEMES)
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 GEOGRAPHIC PRESENCE
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 OCTALSOFT (GLORANT, LLC)
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 GEOGRAPHIC PRESENCE
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 MASTERCONTROL SOLUTIONS, INC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 GEOGRAPHIC PRESENCE
16.6.4 PRODUCT PORTFOLIO
16.6.5 RECENT DEVELOPMENT
16.7 IQVIA INC
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 GEOGRAPHIC PRESENCE
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPMENT
16.8 CLOUDBYZ
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 GEOGRAPHIC PRESENCE
16.8.4 PRODUCT PORTFOLIO
16.8.5 RECENT DEVELOPMENT
16.9 CLINEVO TECHNOLOGIES
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 GEOGRAPHIC PRESENCE
16.9.4 PRODUCT PORTFOLIO
16.9.5 RECENT DEVELOPMENT
16.1 FLORENCE HEALTHCARE
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 GEOGRAPHIC PRESENCE
16.10.4 PRODUCT PORTFOLIO
16.10.5 RECENT DEVELOPMENT
16.11 ORACLE
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 GEOGRAPHIC PRESENCE
16.11.4 PRODUCT PORTFOLIO
16.11.5 RECENT DEVELOPMENT
16.12 ARISGLOBAL LLC
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 GEOGRAPHIC PRESENCE
16.12.4 PRODUCT PORTFOLIO
16.12.5 RECENT DEVELOPMENT
16.13 MONTRIUM INC
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 GEOGRAPHIC PRESENCE
16.13.4 PRODUCT PORTFOLIO
16.13.5 RECENT DEVELOPMENT
16.14 CRUCIAL DATA SOLUTIONS
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 GEOGRAPHIC PRESENCE
16.14.4 PRODUCT PORTFOLIO
16.14.5 RECENT DEVELOPMENT
16.15 KEY2COMPLIANCE AB (SYMBIOTEQ)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 GEOGRAPHIC PRESENCE
16.15.4 PRODUCT PORTFOLIO
16.15.5 RECENT DEVELOPMENT
16.16 DATARIVER S.R.L
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 GEOGRAPHIC PRESENCE
16.16.4 PRODUCT PORTFOLIO
16.16.5 RECENT DEVELOPMENT
16.17 ETHICA CRO
16.17.1 COMPANY SNAPSHOT
16.17.2 REVENUE ANALYSIS
16.17.3 GEOGRAPHIC PRESENCE
16.17.4 PRODUCT PORTFOLIO
16.17.5 RECENT DEVELOPMENT
16.18 TRIALL (CLINBLOCKS B.V.)
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 GEOGRAPHIC PRESENCE
16.18.4 PRODUCT PORTFOLIO
16.18.5 RECENT DEVELOPMENT
16.19 PREMIER CONSULTING
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 GEOGRAPHIC PRESENCE
16.19.4 PRODUCT PORTFOLIO
16.19.5 RECENT DEVELOPMENT
16.2 PHLEXGLOBAL(A PHARMALEX COMPANY)
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 GEOGRAPHIC PRESENCE
16.20.4 PRODUCT PORTFOLIO
16.20.5 RECENT DEVELOPMENT
16.21 SURECLINICAL INC.
16.21.1 COMPANY SNAPSHOT
16.21.2 REVENUE ANALYSIS
16.21.3 GEOGRAPHIC PRESENCE
16.21.4 PRODUCT PORTFOLIO
16.21.5 RECENT DEVELOPMENT
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
17 CONCLUSION
18 QUESTIONNAIRE
19 RELATED REPORTS
20 ABOUT DATA BRIDGE MARKET RESEARCH

Global Electronic Trial Master File Etmf Systems Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Electronic Trial Master File Etmf Systems Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Electronic Trial Master File Etmf Systems Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.