Global Ewing Sarcoma Drug Market
Market Size in USD Million
CAGR :
% 
 USD
285.13 Million
USD
452.40 Million
2024
2032
USD
285.13 Million
USD
452.40 Million
2024
2032
| 2025 –2032 | |
| USD 285.13 Million | |
| USD 452.40 Million | |
|
|
|
|
Ewing Sarcoma Drug Market Size
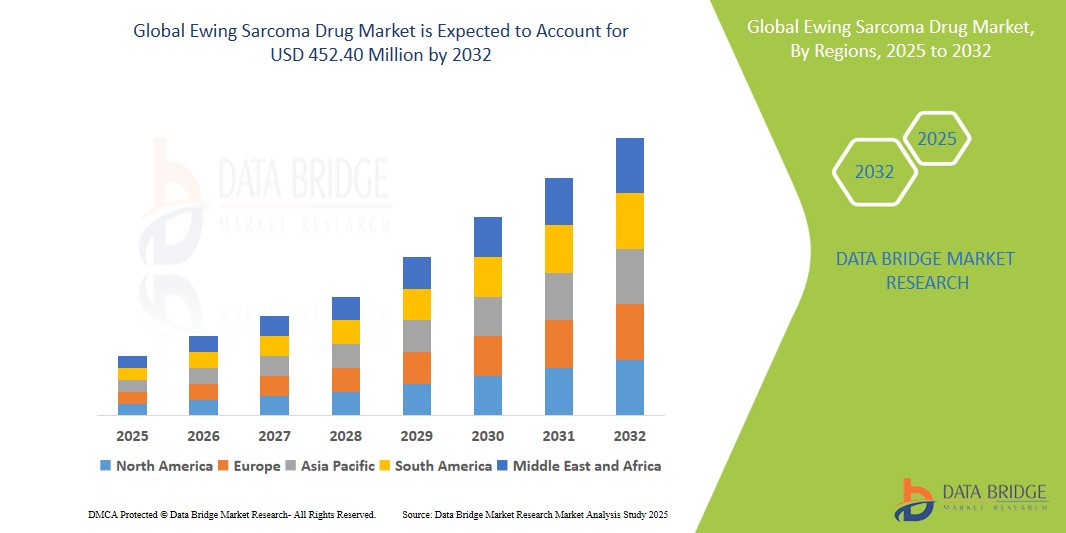
- The Global Ewing Sarcoma Drug Market size was valued at USD 285.13 Million in 2024 and is expected to reach USD 452.40 Million by 2032, at a CAGR of 5.94% during the forecast period
- The market growth is driven by increasing awareness of rare cancers, ongoing advancements in chemotherapy and targeted therapies, and growing investment in orphan drug development
Ewing Sarcoma Drug Market Analysis
- Ewing sarcoma is a rare and aggressive form of cancer that primarily affects children and adolescents. Drug treatments for this condition include chemotherapeutic agents such as Vincristine, Cyclophosphamide, Doxorubicin, Etoposide, Ifosfamide, and Dactinomycin
- The market is fueled by rising incidence rates of Ewing sarcoma globally and the ongoing clinical development of novel targeted therapies and combination drug regimens
- North America holds the largest share in the market due to a well-established oncology treatment landscape, supportive reimbursement frameworks, and strong research infrastructure
- Asia-Pacific is anticipated to witness the fastest growth during the forecast period due to increasing healthcare investments, improving diagnostic capabilities, and rising access to cancer therapies
- The intravenous route of administration dominates the market, accounting for 78.5% of the market share. This is due to its higher efficacy in delivering chemotherapeutic drugs directly into the bloodstream, ensuring better bioavailability and faster therapeutic action compared to other administration routes.
Report Scope and Ewing Sarcoma Drug Market Segmentation
|
Attributes |
Ewing Sarcoma Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ewing Sarcoma Drug Market Trends
“Emerging Targeted Therapies and Personalized Treatment Approaches”
- A key trend in the Ewing sarcoma drug market is the shift toward personalized medicine and targeted therapy approaches, particularly aimed at specific genetic markers such as the EWS-FLI1 fusion gene
- These therapies aim to minimize the systemic toxicity of traditional chemotherapy by selectively targeting tumor cells, improving treatment efficacy while reducing side effects
- For instance, several investigational drugs are being developed to target the EWS-FLI1 oncoprotein, which plays a critical role in the tumorigenesis of Ewing sarcoma. Such novel agents, including small molecule inhibitors and siRNA-based therapies, are in preclinical or early clinical trial stages and show promise for future treatment protocols.
- The development of precision oncology pipelines and biomarker-driven drug discovery platforms is expected to redefine treatment protocols for rare cancers like Ewing sarcoma, representing a transformative trend in the market
Ewing Sarcoma Drug Market Dynamics
Driver
“Increasing Incidence and Awareness of Rare Pediatric Cancers”
- The growing awareness of rare pediatric cancers, combined with an increasing incidence of Ewing sarcoma, particularly among children and adolescents, is driving demand for effective therapies
- Enhanced diagnostic tools and increased surveillance programs have led to earlier detection and more timely treatment, boosting the use of chemotherapeutic and targeted drugs in this segment
- Supportive policies and funding by governments and non-profits for pediatric oncology research are also encouraging pharmaceutical companies to invest in this space
For instance,
- In November 2024, the National Cancer Institute (U.S.) highlighted a 12% rise in childhood sarcoma diagnoses over the past decade, attributing it to improved detection and broader genetic screening programs. These efforts also include grants and incentives for pharmaceutical companies working on pediatric oncology
- As a result, the Ewing sarcoma drug market is experiencing a steady increase in demand, especially for effective, low-toxicity treatment options tailored to young patient
Opportunity
“Incentivized Orphan Drug Designation & Fast-Track Approvals”
- With Ewing sarcoma classified as a rare (orphan) disease, drug developers are benefiting from orphan drug designations that offer regulatory incentives such as market exclusivity, tax credits, and priority reviews
- This designation is fostering innovation in drug development pipelines focused on rare cancers, with several companies entering the space due to the lower risk and higher return potential
- The fast-track designation by agencies like the FDA and EMA facilitates accelerated approval pathways, shortening the time to market and encouraging investment in this niche area
For instance,
- In 2025, Gradalis, Inc. received Orphan Drug Designation from the FDA for its investigational immunotherapy targeting Ewing sarcoma, enabling it to expedite clinical development and secure funding through rare disease support mechanisms
- These regulatory benefits are creating a fertile landscape for biopharma companies to bring novel therapies for Ewing sarcoma to market more efficiently and affordably.
Restraint/Challenge
“Limited Commercial Viability and Small Patient Pool”
- Despite clinical urgency, one of the major challenges in the Ewing sarcoma drug market is its limited patient population, which restricts the commercial viability of new drug development
- As a rare cancer, Ewing sarcoma affects a small demographic, making it less attractive for large-scale pharmaceutical investment due to low expected returns
- Additionally, conducting large, multi-phase clinical trials for rare diseases is often difficult, as patient recruitment is challenging and costly
For instance,
- In 2025, a study published in the Orphanet Journal of Rare Diseases emphasized the difficulties faced by oncology sponsors in enrolling sufficient participants for Phase III Ewing sarcoma trials, often leading to delayed timelines and underpowered studies
- These factors pose a significant barrier to innovation and market expansion, especially for smaller biotech firms lacking the capital to sustain long-term R&D efforts in rare oncology therapeutics
Ewing Sarcoma Drug Market Scope
The market is segmented on the basis drug type, route of administration, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Type |
|
|
By Route of Administration |
|
|
By Distribution Channel |
|
In 2025, the intravenous route of administration is projected to dominate the market with the largest share in the route of administration segment
The intravenous (IV) route of administration segment is expected to dominate the Ewing sarcoma drug market with the largest share of 68.41% in 2025 due to its superior efficacy in delivering chemotherapeutic agents directly into the bloodstream. This method ensures enhanced bioavailability, rapid onset of action, and better therapeutic outcomes, especially critical for aggressive cancers like Ewing sarcoma. The ability to deliver combination chemotherapy regimens intravenously also contributes to its continued market dominance.
The vincristine segment is expected to account for the largest share during the forecast period in the drug type market
In 2025, the vincristine segment is projected to lead the market with the largest share of 24.85% in the drug type segment. As a cornerstone in multi-agent chemotherapy protocols for treating Ewing sarcoma, vincristine's widespread usage and proven clinical efficacy support its leading position. Its synergistic use with other agents like doxorubicin and cyclophosphamide enhances treatment effectiveness, particularly in pediatric and adolescent patient groups, which constitute a major portion of the Ewing sarcoma population.
Ewing Sarcoma Drug Market Regional Analysis
“North America Holds the Largest Share in the Ewing Sarcoma Drug Market”
- North America dominates the global Ewing sarcoma drug market, attributed to well-established healthcare systems, high healthcare spending, and widespread access to advanced oncology treatments
- The U.S. accounts for the largest share in the region, driven by the availability of approved chemotherapeutic drugs, increasing incidence of rare cancers such as Ewing sarcoma, and active participation in clinical trials and research initiatives
- The presence of leading pharmaceutical companies such as Pfizer, Amgen, and Johnson & Johnson, along with robust regulatory frameworks and reimbursement support, further drives market penetration and treatment accessibility
- Furthermore, the availability of specialized cancer treatment centers, increased patient awareness, and strong collaboration between public health agencies and private companies support the continued dominance of the North American market
“Asia-Pacific is Projected to Register the Highest CAGR in the Ewing Sarcoma Drug Market”
- The Asia-Pacific region is projected to witness the fastest growth in the Ewing sarcoma drug market during the forecast period due to increasing healthcare investment, expanding access to oncology care, and rising awareness of rare diseases
- Countries like China, India, and Japan are emerging as key markets due to growing cancer incidence rates, improving diagnostic capabilities, and government support for rare disease treatment initiatives
- Japan, known for its advanced healthcare technologies and precision medicine focus, is increasingly adopting targeted chemotherapeutic and immunotherapeutic approaches for rare cancers, including Ewing sarcoma
- In India and China, rising investments in public healthcare infrastructure, growing pharmaceutical manufacturing capabilities, and greater access to cancer care in urban and semi-urban areas are expected to drive market expansion
- The increasing involvement of international players and rising number of clinical trials in Asia-Pacific further enhance the region’s attractiveness as a high-growth market
Ewing Sarcoma Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Merck & Co., Inc (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Pfizer Inc (U.S.)
- Johnson & Johnson Services, Inc (U.S.)
- Abbott (U.S.)
- Sanofi (France)
- Bausch Health Companies Inc. (Canada)
- Eli Lilly and Company (U.S.)
- AbbVie Inc (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Amgen Inc (U.S.)
- AstraZeneca (UK)
- Bristol-Myers Squibb Company (U.S.)
- Bayer AG (Germany)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- CELGENE CORPORATION (U.S.)
- Eisai Co., Ltd (Japan)
- Gradalis, Inc (U.S.)
- Incyte (U.S.)
Latest Developments in Global Ewing Sarcoma Drug Market
- On November 12, 2024, Actuate Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation to elraglusib, a novel glycogen synthase kinase-3 beta (GSK-3β) inhibitor, for the treatment of Ewing sarcoma (EWS).
- On November 12, 2024, the FDA granted rare pediatric disease designation to elraglusib (9-ING-41), a novel GSK-3β inhibitor, as a potential treatment for patients with Ewing sarcoma.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.