Global Extracellular Vesicles Based Liquid Biopsy Market
Market Size in USD Million
CAGR :
% 
 USD
115.09 Million
USD
539.41 Million
2024
2032
USD
115.09 Million
USD
539.41 Million
2024
2032
| 2025 –2032 | |
| USD 115.09 Million | |
| USD 539.41 Million | |
|
|
|
|
Extracellular Vesicles Based Liquid Biopsy Market Size
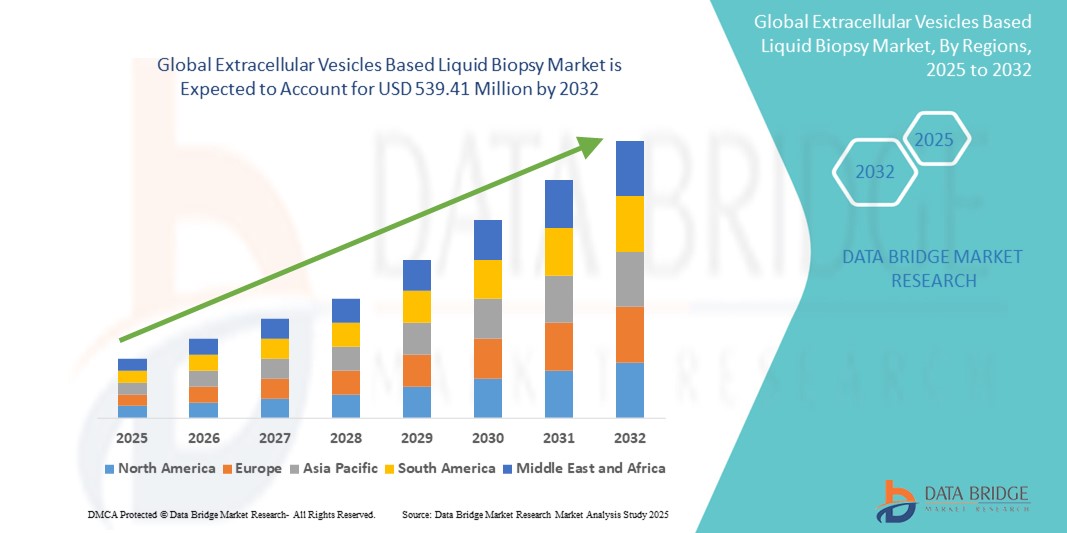
- The global extracellular vesicles based liquid biopsy market size was valued at USD 115.09 Million in 2024 and is expected to reach USD 539.41 Million by 2032, at a CAGR of 21.3% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced diagnostic techniques and continuous technological innovations in molecular diagnostics, particularly in the context of non-invasive cancer detection. The ability of extracellular vesicles (EVs) to carry comprehensive molecular information such as proteins, RNAs, and lipids makes them highly attractive for liquid biopsy applications, promoting their rapid integration in oncology research and clinical diagnostics
- Furthermore, the rising demand for early cancer detection, treatment monitoring, and recurrence surveillance is accelerating the uptake of Extracellular Vesicles Based Liquid Biopsy solutions, significantly boosting the industry's growth. These biopsies offer a safer, less invasive alternative to tissue biopsies, allowing for real-time insights into tumor evolution and heterogeneity. As a result, both academic researchers and pharmaceutical companies are investing heavily in EV-based technologies to improve personalized medicine outcomes
Extracellular Vesicles Based Liquid Biopsy Market Analysis
- Extracellular vesicles (EVs)-based liquid biopsies, offering non-invasive molecular diagnostics, are becoming increasingly crucial in modern healthcare due to their ability to capture disease-specific biomarkers in real-time from various body fluids. This technology is particularly valued for its utility in cancer detection, neurological disorders, and cardiovascular conditions
- The demand for EV-based liquid biopsy solutions is primarily driven by the rising prevalence of chronic diseases, growing adoption of personalized medicine, and increasing need for early, non-invasive diagnostic tools that can monitor disease progression and response to therapy
- North America dominated the extracellular vesicles based liquid biopsy market with the largest revenue share of 43.25% in 2024, fueled by robust R&D investment, early adoption of cutting-edge diagnostic technologies, and strong collaboration between academic institutions and biotech companies. The U.S., in particular, leads the region with a growing number of clinical studies and product developments aimed at validating EV-based diagnostics in oncology and other diseases
- Asia-Pacific is expected to witness the fastest growth in the extracellular vesicles based liquid biopsy market, driven by increasing healthcare expenditure, expanding cancer patient pool, and rapid improvements in biotechnology infrastructure across countries such as China, India, and Japan
- The Isolation segment dominated the extracellular vesicles based liquid biopsy market with a market share of 58.7% in 2024, attributed to its critical role in obtaining high-purity extracellular vesicles (EVs), which ensures superior sample quality and enhances downstream diagnostic accuracy. The increasing demand for standardized and efficient isolation methods across research and clinical applications is further propelling the dominance of this segment in the global extracellular vesicles based liquid biopsy market
Report Scope and Extracellular Vesicles Based Liquid Biopsy Market Segmentation
|
Attributes |
Extracellular Vesicles Based Liquid Biopsy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Extracellular Vesicles Based Liquid Biopsy Market Trends
“Enhanced Efficiency Through AI-Driven Analysis and Automation”
- A significant and accelerating trend in the global extracellular vesicles based liquid biopsy market is the integration of artificial intelligence (AI) and machine learning (ML) technologies into data analysis workflows. This integration is transforming the way liquid biopsy data is processed by enabling rapid, high-throughput interpretation of complex molecular information derived from extracellular vesicles (EVs)
- For instance, AI algorithms are increasingly being employed to identify patterns in EV-derived biomarkers—such as miRNAs, proteins, and nucleic acids—that are indicative of specific cancer types or disease stages. Companies such as Exosome Diagnostics (a Bio-Techne brand) are incorporating AI-based platforms to enhance the precision of their EV-based diagnostic tools
- AI-powered systems help researchers and clinicians reduce false positives and negatives, increasing the clinical validity of EV-based tests. These platforms also support longitudinal patient monitoring by automatically analyzing data trends over time, improving early detection and personalized treatment planning
- In addition, automation technologies integrated with AI are streamlining the entire liquid biopsy workflow, from sample preparation to result reporting. Instruments equipped with smart analytics can perform on-the-fly quality control checks, flagging anomalies and ensuring higher reproducibility across testing environments
- Several academic labs and biotech firms are also using AI models to simulate EV behavior in silico, allowing faster development of new assays and predictive diagnostic tools. This convergence of AI and EV-based liquid biopsy is expected to accelerate translational research and regulatory approval timelines
- The growing demand for AI-enhanced EV liquid biopsy platforms is evident across academic, pharmaceutical, and clinical laboratories, as these institutions seek tools that deliver speed, accuracy, and actionable insights in cancer diagnostics and beyond
Extracellular Vesicles Based Liquid Biopsy Market Dynamics
Driver
“Growing Need Due to Rising Cancer Burden and Demand for Non-Invasive Diagnostics”
- The increasing global burden of cancer and the growing demand for non-invasive diagnostic solutions are major drivers fueling the growth of the Extracellular vesicles based liquid biopsy market. EVs, including exosomes, offer a promising avenue for early cancer detection, treatment monitoring, and prognosis through simple biofluid sampling
- For instance, in April 2024, Exosome Diagnostics, Inc. announced the development of an advanced EV-based assay platform designed to improve early-stage detection of prostate and lung cancers, reinforcing the industry’s focus on precision medicine and non-invasive approaches
- As healthcare systems prioritize early detection and personalized medicine, EV-based liquid biopsies offer advantages such as higher sensitivity, real-time molecular insights, and the ability to detect tumor heterogeneity—giving them a competitive edge over traditional biopsies
- Furthermore, the rising adoption of EVs in clinical trials, translational research, and companion diagnostics is driving widespread interest among pharmaceutical companies and research institutes, thereby accelerating innovation and commercial uptake
- The ease of sample collection (e.g., blood, urine, saliva), combined with minimal patient discomfort and reduced risk of complications, makes EV-based liquid biopsies increasingly preferred in oncology and neurological disease diagnostics. As a result, the market is experiencing robust growth across academic centers, diagnostics labs, and biopharma companies
Restraint/Challenge
“Standardization Issues and High Development Costs”
- Despite their immense potential, challenges surrounding standardization and clinical validation of EV-based assays are hindering the rapid commercialization of liquid biopsy solutions. Differences in isolation techniques, quantification standards, and biomarker reproducibility remain significant concerns
- For instance, variability in EV purification protocols across research labs can lead to inconsistent diagnostic results, creating regulatory hurdles and slowing clinical adoption
- In addition, the high cost of EV-based diagnostic development, including the need for sophisticated instruments and skilled personnel, limits accessibility in resource-constrained settings. This poses a major barrier to widespread market penetration, particularly in low- and middle-income countries
- To overcome these barriers, companies such as Bio-Techne and QIAGEN are investing in scalable, standardized EV isolation kits and detection platforms, aiming to improve reproducibility and regulatory compliance
- Further, increasing collaboration between industry and regulatory bodies such as the FDA and EMA is critical to establishing clear guidelines for EV-based diagnostic approvals, which will be essential to unlocking the full market potential
- Continued innovation, regulatory alignment, and affordability-focused product development will be key to addressing these challenges and sustaining long-term market growth
Extracellular Vesicles Based Liquid Biopsy Market Scope
The market is segmented on the basis of offering, technology, workflow, and end user.
- By Offering
On the basis of offering, the extracellular vesicles based liquid biopsy market is segmented into kits and assays, services, and instruments. The kits and assays segment dominated the market with a 46.3% share in 2024, driven by the wide adoption of standardized and user-friendly kits for EV isolation and biomarker detection across research and clinical applications.
The services segment is expected to register the fastest CAGR of 20.9% from 2025 to 2032, owing to the growing demand for outsourced liquid biopsy testing, especially among small- and mid-sized research entities and clinical labs.
- By Technology
On the basis of technology, the extracellular vesicles based liquid biopsy market is segmented into isolation and analysis. The isolation segment held the largest share of 58.7% in 2024, attributed to the critical role of high-purity isolation in ensuring sample quality and downstream diagnostic accuracy.
The analysis segment is anticipated to grow at the highest CAGR of 18.4% from 2025 to 2032, driven by rapid technological advancements in EV content profiling, including nucleic acids, proteins, and metabolites.
- By Workflow
On the basis of workflow, the extracellular vesicles based liquid biopsy market is segmented into sample preparation, sequencing, and data analysis. The sample preparation segment accounted for the largest revenue share of 41.6% in 2024, due to the need for reproducible, contamination-free sample handling in clinical EV-based assays.
The data analysis segment is projected to expand at the fastest CAGR of 21.2% from 2025 to 2032, owing to increased adoption of AI-powered analytics, machine learning algorithms, and cloud-based platforms to interpret complex multi-omic EV data.
- By End User
On the basis of end user, the extracellular vesicles based liquid biopsy market is segmented into academic and research institutes, clinical laboratories, and pharmaceutical and biotechnology companies. The academic and research institutes segment held the largest share of 44.9% in 2024, supported by rising government and institutional funding for EV research in cancer, neurology, and cardiovascular diagnostics.
The pharmaceutical and biotechnology companies segment is expected to witness the highest CAGR of 19.6% from 2025 to 2032, as these companies increasingly adopt EV-based liquid biopsy tools for non-invasive drug response monitoring and biomarker discovery in personalized medicine.
Extracellular Vesicles Based Liquid Biopsy Market Regional Analysis
- North America dominated the extracellular vesicles based liquid biopsy market with the largest revenue share of 43.25% in 2024, driven by increasing investment in cancer diagnostics, rapid adoption of precision medicine, and the presence of key biotechnology firm
- Favorable reimbursement policies and strong support for liquid biopsy research in the U.S. have further accelerated the uptake of EV-based diagnostic platforms
- The region’s growth is further propelled by an established healthcare infrastructure, rising awareness among clinicians about the clinical utility of EVs, and robust collaborations between academia and industry aimed at developing advanced non-invasive cancer diagnostics
U.S. Extracellular Vesicles Based Liquid Biopsy Market Insight
The U.S. extracellular vesicles based liquid biopsy market accounted for 78.3% of the North American revenue share in 2024, owing to strong demand for early cancer detection tools, widespread use of personalized medicine, and increasing patient preference for non-invasive diagnostic methods. The presence of major players such as Bio-Techne, Exosome Diagnostics, and Thermo Fisher Scientific, coupled with aggressive R&D funding, continues to drive product innovation and clinical trial expansion in the country.
Europe Extracellular Vesicles Based Liquid Biopsy Market Insight
The Europe extracellular vesicles based liquid biopsy market is projected to grow at a substantial CAGR during the forecast period, bolstered by rising cancer incidence, growing focus on minimally invasive diagnostics, and supportive regulatory frameworks. Countries such as Germany, the U.K., and France are increasingly integrating EV-based assays into research and diagnostic pipelines, particularly in academic and oncology-focused clinical centers.
U.K. Extracellular Vesicles Based Liquid Biopsy Market Insight
The U.K. extracellular vesicles based liquid biopsy market is anticipated to grow significantly owing to the growing implementation of precision oncology practices and robust government funding for translational research. Increased awareness of early cancer detection and strong adoption of liquid biopsy tests in NHS pilot programs are expected to further support the market expansion.
Germany Extracellular Vesicles Based Liquid Biopsy Market Insight
The Germany extracellular vesicles based liquid biopsy market is expanding due to its advanced biotechnology sector, rigorous healthcare standards, and focus on innovation. The country is a key hub for clinical research and EV-based biomarker validation studies. The demand for cost-effective and scalable diagnostic solutions is creating opportunities for partnerships and academic-industry collaboration.
Asia-Pacific Extracellular Vesicles Based Liquid Biopsy Market Insight
The Asia-Pacific extracellular vesicles based liquid biopsy market is projected to grow at the fastest CAGR of 24% from 2025 to 2032, driven by rising cancer prevalence, improving healthcare infrastructure, and government-led initiatives promoting early disease detection. Countries such as China, Japan, and India are investing heavily in biopharma research and expanding access to advanced diagnostics, making EV-based liquid biopsies increasingly viable and accessible.
Japan Extracellular Vesicles Based Liquid Biopsy Market Insight
The Japan extracellular vesicles based liquid biopsy market is benefiting from a strong focus on high-tech healthcare innovations and a growing aging population. The demand for early, minimally invasive diagnostics is rising, particularly for cancers such as gastric, colorectal, and lung. Government-backed precision medicine programs are expected to further accelerate market growth.
China Extracellular Vesicles Based Liquid Biopsy Market Insight
The China extracellular vesicles based liquid biopsy market accounted for the largest revenue share within the Asia-Pacific market in 2024, supported by rapid urbanization, increasing cancer screening programs, and expanding investments in biotechnology. Domestic companies are making strides in EV-based platform development, and the government's push toward smart healthcare and local innovation is strengthening the market outlook.
Extracellular Vesicles Based Liquid Biopsy Market Share
The extracellular vesicles based liquid biopsy industry is primarily led by well-established companies, including:
- Bio-Techne Corporation (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN N.V. (Netherlands)
- Exosome Diagnostics, Inc. (U.S.)
- Aethlon Medical, Inc. (U.S.)
- System Biosciences, LLC (U.S.)
- NanoSomix, Inc. (U.S.)
- Malvern Panalytical Ltd. (U.K.)
- Norgen Biotek Corp. (Canada)
- Hitachi Chemical Co., Ltd. (Japan)
- Lonza Group Ltd. (Switzerland)
- Codiak BioSciences, Inc. (U.S.)
- NX Pharmagen (South Korea)
- Stemcell Technologies Inc. (Canada)
- Aruna Bio (U.S.)
- Evomic Science LLC (U.S.)
- Izon Science Ltd. (New Zealand)
- Exovita Biosciences (U.S.)
- Anergis SA (Switzerland)
- System Biosciences (SBI) (U.S.)
Latest Developments in Global Extracellular Vesicles Based Liquid Biopsy Market
- In January 2022, QIAGEN announced its new Biotech Grants program winners, an initiative driven by the company's commitment to building strong partnerships with biotechnology and pharma businesses and helping them succeed in the market. This has helped the company in revenue growth
- In January 2022, Exact Sciences Corporation announced the acquisition of Prevention Genetics, a genetic testing laboratory, to complement its advanced cancer diagnostics portfolio and support its entrance into hereditary cancer testing (HCT)
- In July 2024, Mursla Bio announced the launch of its novel extracellular vesicle (EV)-based diagnostic platform aimed at enhancing early detection of diseases such as cancer through liquid biopsy. The new platform enables ultra-sensitive and high-specificity analysis of biomarkers using EVs from blood samples, marking a significant step forward in non-invasive diagnostics. This innovation is expected to strengthen Mursla Bio’s position in the precision medicine market and drive revenue growth
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.