Global Familial Chylomicronemia Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
14.23 Million
USD
20.83 Million
2024
2032
USD
14.23 Million
USD
20.83 Million
2024
2032
| 2025 –2032 | |
| USD 14.23 Million | |
| USD 20.83 Million | |
|
|
|
Familial Chylomicronemia Syndrome Market Analysis
The market for Familial Chylomicronemia Syndrome (FCS) is experiencing gradual growth, driven by increasing awareness and advancements in treatment options. Despite being a rare condition, FCS presents significant unmet medical needs, particularly due to its severe manifestations, including recurrent pancreatitis and cardiovascular complications. The lack of approved, standardized therapies for FCS patients positions the market as a promising area for pharmaceutical development.
Current treatment strategies focus on triglyceride reduction through lifestyle modifications, such as dietary changes and medications such as fibrates, but these are often inadequate in controlling symptoms. As a result, several biotech and pharmaceutical companies are investing in research to develop more effective treatments, including gene therapies, enzyme replacement therapies, and novel lipid-lowering agents. These innovations aim to address the root causes of FCS and offer better long-term management options for patients.
The rise in genetic testing, which allows for earlier detection and more personalized treatment plans, further boosts the market demand. The increasing recognition of FCS as a distinct and serious disorder among healthcare providers is contributing to earlier diagnosis and improved patient outcomes. As the market continues to evolve, the availability of specialized treatments and improved diagnostic tools will likely drive significant growth in the FCS market, presenting opportunities for both pharmaceutical companies and healthcare providers.
Familial Chylomicronemia Syndrome Market Size
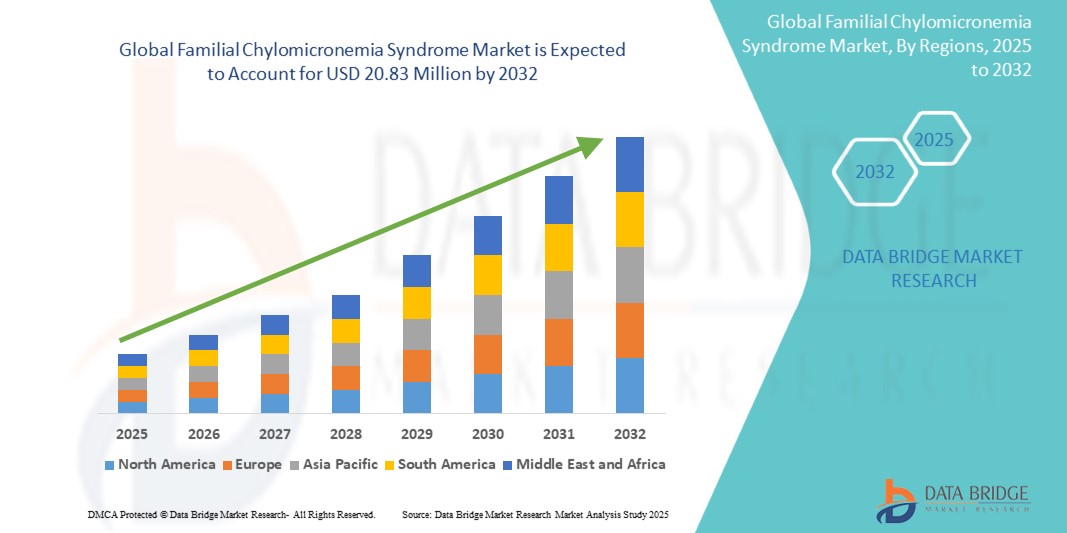
The global Familial Chylomicronemia Syndrome market size was valued at USD 14.23 million in 2024 and is projected to reach USD 20.83 million by 2032, with a CAGR of 4.88% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Familial Chylomicronemia Syndrome Market Trends
“Growing Focus on Genetic-Based Therapies”
One key trend in the Familial Chylomicronemia Syndrome (FCS) market is the growing focus on genetic-based therapies. With FCS being a rare genetic disorder, advancements in gene therapy, enzyme replacement therapy, and targeted lipid-lowering treatments are gaining momentum. These therapies aim to address the root cause of the disease, offering more effective and personalized management options compared to traditional treatments that primarily focus on symptom control, such as dietary changes and triglyceride-lowering medications.
Gene therapy, in particular, holds promise by potentially correcting the underlying genetic mutations responsible for FCS, providing a long-term solution. In addition, innovations in genetic testing enable earlier and more accurate diagnoses, leading to quicker interventions and improved patient outcomes. As the understanding of the genetic mechanisms behind FCS improves, the market is shifting towards more tailored treatments, presenting opportunities for growth and better quality of life for patients.
Report Scope and Familial Chylomicronemia Syndrome Market Segmentation
|
Attributes |
Familial Chylomicronemia Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
AstraZeneca (U.K.), Arrowhead Pharmaceuticals Inc. (U.S.), Ionis Pharmaceuticals (U.S.), PTC Therapeutics, Inc. (U.S.) and Regeneron Pharmaceuticals Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Familial Chylomicronemia Syndrome Market Definition
Familial Chylomicronemia Syndrome (FCS) is a rare, genetic disorder characterized by extremely high levels of triglycerides in the blood. This condition results from mutations in genes responsible for lipid metabolism, particularly the LPL (lipoprotein lipase) gene, which impairs the body's ability to break down triglycerides. As a result, chylomicrons, which are particles responsible for transporting triglycerides, accumulate in the bloodstream.
The elevated triglyceride levels can lead to severe health complications, including acute pancreatitis, which can be life-threatening, as well as chronic issues such as organ damage due to the persistent buildup of fat. Symptoms of FCS often include recurrent abdominal pain, nausea, and vomiting, which are linked to pancreatitis. FCS is typically diagnosed through genetic testing and lipid profile assessments. Management primarily focuses on lowering triglyceride levels through dietary changes, medications, and in some cases, more specialized treatments.
Familial Chylomicronemia Syndrome Market Dynamics
Drivers
- Advancements in Targeted Therapies
The development of novel, targeted therapies is a major driver of growth in the Familial Chylomicronemia Syndrome (FCS) market. Traditional treatments, which primarily focus on triglyceride reduction through lifestyle modifications and existing medications, often fail to adequately address the underlying genetic causes of FCS. New treatments, such as olezarsen (TRYNGOLZA) by Ionis Pharmaceuticals and plozasiran by Arrowhead Pharmaceuticals, have emerged as more effective options. Olezarsen, for instance, received FDA approval in December 2024, offering a therapy designed to specifically target lipid metabolism in FCS patients. Similarly, plozasiran has received positive regulatory attention, with a PDUFA action date set for November 2025. These therapies, which promise better management of triglyceride levels, reflect a significant shift toward more precise and personalized treatment options. The increasing availability of such therapies is expected to drive market growth by offering better long-term outcomes for patients and increasing demand for specialized FCS care.
- Increased Awareness and Early Diagnosis
Improved awareness and advancements in genetic testing have led to earlier and more accurate diagnosis of Familial Chylomicronemia Syndrome (FCS), which is a critical driver of market growth. As healthcare providers become more familiar with the condition and its symptoms, there has been an uptick in the diagnosis of FCS, allowing for timely interventions before severe complications, such as acute pancreatitis, occur. Genetic testing, which helps identify the underlying mutations causing FCS, has also made early detection more feasible, enabling earlier treatment with emerging therapies. The recognition of FCS as a distinct disorder is driving healthcare professionals to adopt more aggressive management approaches. This heightened awareness and diagnosis rate are expanding the patient pool in need of specialized treatments, thus contributing to a growing demand for FCS-specific therapies and further driving market expansion.
Opportunities
- Expansion of Gene Therapy
One significant opportunity in the Familial Chylomicronemia Syndrome (FCS) market lies in the development and application of gene therapies. Given that FCS is caused by genetic mutations affecting lipid metabolism, gene therapy presents a promising long-term solution by directly addressing the root cause of the condition. Companies such as Arrowhead Pharmaceuticals with their investigational drug plozasiran, and ongoing research into gene-editing technologies, could significantly change the treatment landscape for FCS. Gene therapies that aim to repair or replace defective genes offer the potential for a more permanent solution, reducing the need for ongoing treatments and improving long-term patient outcomes. If successful, such treatments would not only provide better management but could also reduce the overall cost of lifelong care. This opportunity in gene therapy holds the potential to revolutionize FCS treatment, driving market growth by attracting investment, increasing demand for advanced therapies, and positioning the market for significant expansion.
- Increased Focus on Rare Disease Initiatives and Orphan Drug Designations
Another key opportunity in the FCS market is the growing emphasis on rare disease initiatives and the increasing number of Orphan Drug Designations granted by regulatory bodies. With FCS being a rare and severe genetic disorder, regulatory agencies such as the FDA and the European Medicines Agency (EMA) offer incentives, such as faster approvals and extended market exclusivity, to companies developing therapies for these conditions. For instance, Ionis Pharmaceuticals' olezarsen received Orphan Drug designation, significantly streamlining the development process and enhancing its market potential. These initiatives help reduce the cost and time associated with developing treatments for rare diseases, encouraging more pharmaceutical companies to invest in the FCS market. The support for orphan drugs creates a favorable environment for innovation, leading to the development of more specialized and effective treatments, thus driving market growth and expanding treatment options for patients with FCS.
Restraints/Challenges
- Regulatory Hurdles and Lengthy Approval Process
A key restraint in the Familial Chylomicronemia Syndrome (FCS) market is the regulatory hurdles and lengthy approval process for new treatments. FCS is a rare disease, and as such, the development of therapies often requires extensive clinical trials, which can take years to complete due to the limited patient population. Regulatory agencies such as the FDA and EMA require significant evidence of safety and efficacy before granting approval, which can delay the availability of new treatments. For instance, Arrowhead Pharmaceuticals' investigational drug plozasiran had to undergo extensive clinical trials before receiving NDA submission, with the FDA PDUFA action date set for 2025. The time and resources needed to navigate regulatory processes can increase development costs and slow down the market entry of potential therapies, limiting the growth of available treatment options for FCS patients.
- Lack of Long-Term Efficacy Data
One of the significant challenges facing the Familial Chylomicronemia Syndrome (FCS) market is the lack of long-term efficacy data for newer therapies. Although treatments such as olezarsen (TRYNGOLZA) and plozasiran show promise in clinical trials, the absence of long-term studies on their effectiveness and safety limits confidence in their sustained use. Since FCS is a lifelong condition, patients require therapies that not only work in the short term but also provide lasting benefits without long-term side effects. The uncertainty surrounding the long-term effects of these treatments may deter healthcare providers and payers from fully embracing them. As a result, the market faces slower adoption rates, and there may be hesitancy from both patients and healthcare professionals to commit to new therapies without more comprehensive long-term data. This challenge hampers market growth by limiting widespread acceptance of emerging treatments.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Familial Chylomicronemia Syndrome Market Scope
The market is segmented on the basis of therapeutic approach, treatment stage, technology and diagnostic tools, and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Therapeutic Approach
- Genetic Therapies
- Conventional Pharmacotherapy
- Nutritional Management
- Supportive Therapies
Treatment Stage
- Early Intervention Strategies
- Chronic Management Therapies
- Acute Complication Treatments
Technology and Diagnostic Tools
- Genetic Testing
- Lipid Profiling
- Imaging Techniques
- Point-of-Care Diagnostics
Application
- Hospital Pharmacies
- Retail Pharmacies
Familial Chylomicronemia Syndrome Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, therapeutic approach, treatment stage, technology and diagnostic tools, and application as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Familial Chylomicronemia Syndrome (FCS) market due to the presence of advanced healthcare infrastructure, increased awareness, and early diagnosis of rare diseases. The region's significant investment in rare disease research and regulatory support for orphan drugs further accelerates the market growth. The approval of treatments such as olezarsen and plozasiran in the U.S., coupled with a high prevalence of FCS diagnoses, positions North America as the leading market for FCS therapies.
Asia-Pacific is expected to exhibit the highest growth rate in the Familial Chylomicronemia Syndrome (FCS) market. This growth is driven by improving healthcare infrastructure, increasing awareness of rare diseases, and advancements in genetic testing across countries such as Japan, China, and India. In addition, the growing adoption of orphan drug policies and increasing healthcare spending are expected to fuel market expansion. The rising diagnosis rates and availability of new treatments will further accelerate market growth in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Familial Chylomicronemia Syndrome Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Familial Chylomicronemia Syndrome Market Leaders Operating in the Market Are:
- AstraZeneca (U.K.)
- Arrowhead Pharmaceuticals Inc. (U.S.),
- Ionis Pharmaceuticals (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
Latest Developments in Familial Chylomicronemia Syndrome Market
- In January 2025, Arrowhead Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for its investigational treatment, plozasiran, for Familial Chylomicronemia Syndrome, a rare and severe genetic disorder. The FDA has set a Prescription Drug User Fee Act (PDUFA) action date of November 18, 2025, and has stated that it does not plan to convene an advisory committee meeting for the application.
- In December 2024, Ionis Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved TRYNGOLZA (olezarsen) as an adjunct to diet for reducing triglyceride levels in adults with Familial Chylomicronemia Syndrome, a rare genetic condition causing severe hypertriglyceridemia that can result in potentially life-threatening acute pancreatitis.
- In February 2024, Ionis Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug designation to its investigational medicine, olezarsen, for the treatment of Familial Chylomicronemia Syndrome (FCS), a rare genetic disorder marked by extremely high triglyceride levels and recurrent acute pancreatitis.
- In January 2022, PTC Therapeutics, Inc. announced that Waylivra (volanesorsen) had been granted Category 1 classification by the Câmara de Regulação do Mercado de Medicamentos (CMED), the Drug Market Regulation Chamber, in Brazil. Waylivra is the only approved treatment for Familial Chylomicronemia Syndrome (FCS) in the country.
- In August 2021, PTC Therapeutics, Inc. announced that the Brazilian Health Regulatory Agency, ANVISA (Agência Nacional de Vigilância Sanitária), had approved Waylivra (volanesorsen) as the first treatment for Familial Chylomicronemia Syndrome (FCS) in Brazil. FCS is a rare genetic disorder that imposes a significant disease burden on patients, including the risk of potentially fatal pancreatitis and long-term complications due to permanent organ damage.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.