Global Fetal Valproate Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
1.32 Billion
USD
1.82 Billion
2024
2032
USD
1.32 Billion
USD
1.82 Billion
2024
2032
| 2025 –2032 | |
| USD 1.32 Billion | |
| USD 1.82 Billion | |
|
|
|
|
Fetal Valproate Syndrome Market Size
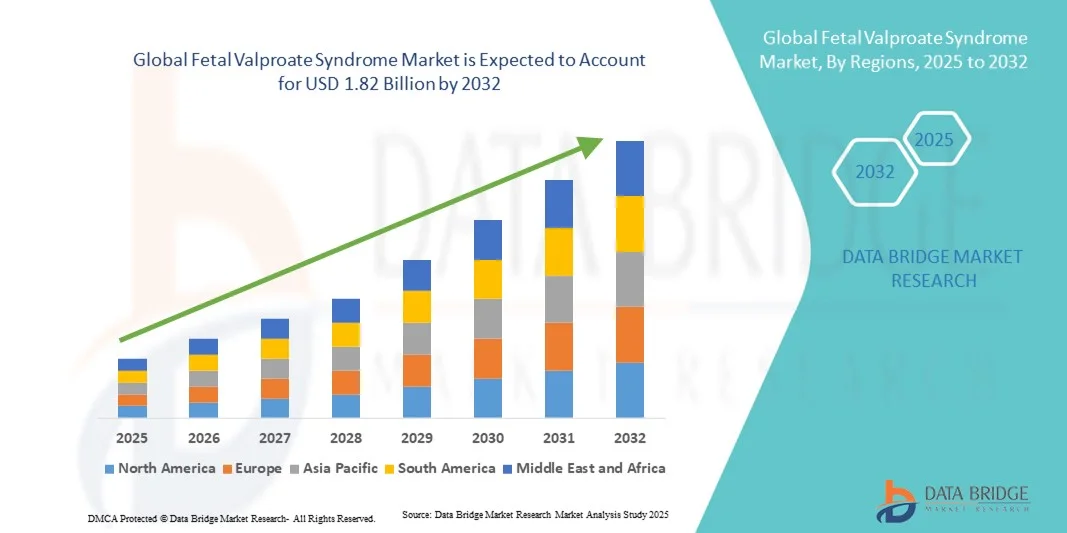
- The global fetal valproate syndrome market size was valued at USD 1.32 billion in 2024 and is expected to reach USD 1.82 billion by 2032, at a CAGR of 4.10% during the forecast period
- The market growth is largely driven by the rising prevalence of rare congenital disorders and increasing awareness regarding the teratogenic effects of valproate exposure during pregnancy. Advancements in genetic testing and prenatal screening are improving early diagnosis and management outcomes for affected infants, further contributing to market expansion
- Furthermore, growing research efforts to understand the molecular mechanisms of fetal valproate syndrome (FVS) and the introduction of supportive care and rehabilitation programs are significantly boosting the demand for specialized treatment solutions, thereby driving the overall growth of the fetal valproate syndrome market
Fetal Valproate Syndrome Market Analysis
- Fetal Valproate Syndrome (FVS), a rare congenital condition caused by prenatal exposure to valproic acid, is gaining increasing attention in the global healthcare landscape due to its growing recognition among clinicians and public health authorities. The condition’s rising diagnosis rates and the need for long-term management of neurological and developmental complications are driving the demand for improved treatment and prevention strategies
- The growing focus on maternal health safety, coupled with government-led awareness programs about the teratogenic risks of valproate during pregnancy, is significantly contributing to market growth. Pharmaceutical companies and research organizations are increasingly investing in the development of safer antiepileptic drug formulations and genetic screening tools to minimize fetal exposure risks and improve patient outcomes
- North America dominated the fetal valproate syndrome market with the largest revenue share of 39.6% in 2024, driven by increasing awareness of the risks associated with valproate exposure during pregnancy, strong regulatory oversight, and the presence of advanced healthcare infrastructure. The U.S. continues to lead the region due to active patient advocacy, ongoing clinical research on prenatal drug safety, and government initiatives promoting early diagnosis and management of congenital disorders
- Asia-Pacific is expected to be the fastest-growing region in the fetal valproate syndrome market during the forecast period, projected to expand at a CAGR fueled by improving maternal healthcare systems, growing public health campaigns, and rising investment in genetic and prenatal diagnostics across countries such as India, Japan, and China
- The therapies segment dominated the market in 2024, accounting for a significant 65.8% revenue share, driven by the widespread use of multidisciplinary therapeutic interventions such as speech therapy, occupational therapy, and behavioral therapy to manage developmental, neurological, and motor deficits in affected children
Report Scope and Fetal Valproate Syndrome Market Segmentation
|
Attributes |
Fetal Valproate Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Fetal Valproate Syndrome Market Trends
Advancements in Genetic Screening and AI-Driven Diagnostics
- A significant and accelerating trend in the global fetal valproate syndrome (FVS) market is the integration of artificial intelligence (AI) and advanced genetic screening tools to improve early diagnosis and clinical decision-making. This technological evolution is transforming prenatal care by enhancing precision, reducing diagnostic delays, and supporting better risk stratification for affected pregnancies
- For instance, in January 2024, researchers at King’s College London introduced an AI-based fetal imaging system capable of detecting craniofacial and neurological abnormalities linked to FVS with greater accuracy than conventional ultrasound. Such innovations are fostering earlier and more reliable detection
- AI and machine learning are increasingly being utilized to analyze large genomic datasets, enabling clinicians to identify high-risk pregnancies based on maternal medication history and genetic predispositions. This aids in predicting adverse outcomes and optimizing prenatal management strategies
- Furthermore, digital health platforms that combine AI-driven analytics with maternal electronic health records (EHRs) are enabling real-time monitoring of fetal growth and developmental anomalies associated with valproate exposure
- The growing emphasis on precision medicine and personalized obstetric care has encouraged healthcare providers to incorporate AI-enhanced genetic testing into prenatal programs. Companies and research institutes are collaborating to integrate AI with genomic tools for better diagnosis and treatment planning
- This convergence of technology and maternal-fetal medicine is not only improving diagnostic reliability but also supporting data-driven policy development regarding the safe use of valproate in women of childbearing potential
- The trend reflects a global shift toward proactive, data-backed approaches in fetal health management, enabling early intervention and better clinical outcomes for affected infants
Fetal Valproate Syndrome Market Dynamics
Driver
Growing Awareness, Regulatory Initiatives, and Advancements in Maternal Health Management
- The increasing awareness of medication-related teratogenic risks, coupled with regulatory actions to restrict valproate prescriptions during pregnancy, serves as a major driver for the global FVS market. Health authorities and advocacy groups are intensifying educational campaigns to promote safer treatment alternatives for women of reproductive age
- For instance, in April 2024, the European Medicines Agency (EMA) strengthened its risk minimization measures for valproate-containing products, mandating visual warnings and patient safety leaflets with every prescription to reduce fetal exposure
- The growing burden of developmental disorders associated with in-utero valproate exposure is propelling research efforts to enhance prenatal screening and postnatal therapeutic options
- Increasing investments in fetal medicine, coupled with multidisciplinary care models that integrate obstetricians, neurologists, and geneticists, are improving diagnostic and treatment standards
- Furthermore, the emergence of national pregnancy registries, such as those in the U.K. and Japan, is contributing to better data collection and outcome tracking, supporting targeted prevention initiatives
- Rising government support for maternal health, combined with technological progress in prenatal imaging and laboratory testing, continues to drive the adoption of advanced FVS diagnostic programs worldwide
- The collaboration between pharmaceutical companies and research institutions to identify non-teratogenic alternatives for epilepsy and bipolar disorder patients reinforces the broader shift toward safety-conscious drug use during pregnancy
Restraint/Challenge
Diagnostic Complexity, Limited Awareness, and Ethical Challenges in Drug Regulation
- The accurate diagnosis of fetal valproate syndrome remains challenging due to overlapping clinical features with other neurodevelopmental disorders, leading to frequent underdiagnosis and delayed interventions
- For instance, many cases go unrecognized in early childhood due to subtle phenotypic traits, contributing to inconsistent epidemiological data across regions
- Limited awareness among healthcare providers, especially in low- and middle-income countries, hinders timely identification and appropriate management of FVS-affected children
- Moreover, ethical and legal complexities surrounding maternal medication use and pharmaceutical liability create barriers for transparent reporting and regulatory enforcement.
- The lack of standardized diagnostic criteria and globally harmonized guidelines also restricts research progress and clinical comparability between studies
- High costs associated with advanced genetic testing and limited insurance coverage pose financial challenges for expectant mothers in developing regions
- While major drug regulators have implemented strict labeling requirements, compliance monitoring at the prescriber level remains inconsistent
- Overcoming these challenges requires sustained awareness campaigns, improved access to prenatal screening, and ethical frameworks that balance patient confidentiality with public health safety
- Strengthening physician training, expanding research funding for alternative therapies, and enhancing pharmacovigilance mechanisms will be critical to ensuring continued progress in preventing and managing Fetal Valproate Syndrome globally
Fetal Valproate Syndrome Market Scope
The market is segmented on the basis of symptoms, gender, and treatment.
- By Symptoms
On the basis of symptoms, the Fetal Valproate Syndrome (FVS) market is segmented into characteristic facial features, spina bifida, congenital heart defects, cleft lip and/or cleft palate, genital abnormalities, skeletal abnormalities, and developmental delay. The developmental delay segment dominated the largest market revenue share of 38.4% in 2024, driven by the high prevalence of cognitive and neurological impairments observed in children exposed to valproate during pregnancy. The growing number of developmental disorder diagnoses and the increasing demand for long-term rehabilitation and therapy services have further contributed to the segment’s dominance. Advancements in early childhood intervention programs and government-supported developmental monitoring initiatives also play a critical role in sustaining market growth. Furthermore, the availability of specialized neurodevelopmental treatment centers and the rising adoption of early intervention therapies are enhancing diagnosis and management outcomes for affected children.
The spina bifida segment is projected to witness the fastest growth rate of 21.1% from 2025 to 2032, attributed to growing awareness of neural tube defects and improved prenatal diagnostic techniques such as high-resolution fetal ultrasound and maternal serum alpha-fetoprotein (AFP) screening. Increasing government emphasis on maternal health safety and the introduction of preventive care programs for high-risk pregnancies are further propelling growth. Moreover, technological advances in fetal surgery and postnatal corrective procedures are improving survival rates and clinical outcomes, encouraging healthcare providers to expand early detection and treatment services globally.
- By Gender
On the basis of gender, the Fetal Valproate Syndrome market is segmented into male and female. The male segment accounted for the largest market revenue share of 54.7% in 2024, as studies indicate that male infants have a higher likelihood of developing neurological and behavioral complications related to FVS exposure. The dominance of this segment is supported by rising awareness and screening for autism spectrum disorders and attention deficit hyperactivity disorder (ADHD), which are more frequently diagnosed in male children with prenatal valproate exposure. Increasing access to developmental health programs and diagnostic facilities in hospitals and pediatric centers also strengthens the market presence of this segment. Furthermore, healthcare professionals are increasingly adopting gender-based risk assessment models to identify at-risk male infants early, enhancing diagnostic precision and care outcomes.
The female segment is expected to register the fastest CAGR of 20.4% from 2025 to 2032, driven by growing attention to congenital and reproductive health abnormalities among female infants affected by FVS. Rising inclusion of female-specific studies in teratogenic research, alongside expanding genetic testing programs, is fostering earlier diagnosis and targeted management approaches. Furthermore, increasing advocacy for maternal and female child health in both developed and emerging regions is supporting healthcare access, contributing to the segment’s accelerating growth trajectory during the forecast period.
- By Treatment
On the basis of treatment, the Fetal Valproate Syndrome market is segmented into therapies and surgery. The therapies segment dominated the market in 2024, accounting for a significant 65.8% revenue share, driven by the widespread use of multidisciplinary therapeutic interventions such as speech therapy, occupational therapy, and behavioral therapy to manage developmental, neurological, and motor deficits in affected children. The segment’s growth is bolstered by the availability of advanced rehabilitation centers, increasing insurance coverage for therapy services, and heightened government support for developmental disorder programs. Furthermore, continuous innovation in therapy delivery methods—such as virtual and telehealth-based models—has made these treatments more accessible to families across both urban and rural areas. The rise in pediatric neurorehabilitation initiatives and partnerships between public health agencies and educational institutions is further amplifying adoption.
The surgery segment is anticipated to witness the fastest growth rate of 19.8% from 2025 to 2032, primarily due to the rising incidence of structural defects such as spina bifida, cleft lip and palate, and congenital heart abnormalities among FVS-affected infants. Advancements in fetal and neonatal surgical techniques, coupled with better perioperative care and post-surgical rehabilitation, are driving this segment’s rapid expansion. Increasing investment in pediatric surgical infrastructure and the presence of specialized children’s hospitals in developed regions are also boosting market demand. Moreover, ongoing clinical research into minimally invasive fetal repair procedures and improved prenatal imaging technologies is expected to further accelerate growth in this segment during the forecast period.
Fetal Valproate Syndrome Market Regional Analysis
- North America dominated the fetal valproate syndrome market with the largest revenue share of 39.6% in 2024
- Driven by increasing awareness of the risks associated with valproate exposure during pregnancy, strong regulatory oversight, and the presence of advanced healthcare infrastructure
- The region benefits from early diagnosis initiatives, multidisciplinary treatment centers, and active collaborations between healthcare organizations and patient advocacy groups
U.S. Fetal Valproate Syndrome Market Insight
The U.S. fetal valproate syndrome market captured the largest revenue share of in 2024 within North America, fueled by active patient awareness campaigns, stringent FDA monitoring on valproate prescriptions, and growing clinical research focused on teratogenic risk mitigation. The country’s robust healthcare infrastructure and presence of leading genetic testing companies further support early identification and better management of the condition. Additionally, increased collaboration between pharmaceutical firms and research institutes to develop safer antiepileptic alternatives continues to drive market growth.
Europe Fetal Valproate Syndrome Market Insight
The Europe Fetal Valproate Syndrome market is projected to expand at a steady CAGR during the forecast period, supported by heightened awareness of drug safety in pregnancy and strong government action in countries such as the U.K., France, and Germany. Regulatory agencies, including the EMA, have enforced strict labeling and prescription guidelines for valproate-based drugs, encouraging better maternal counseling. Furthermore, expanding genetic counseling services and neonatal care programs are contributing to improved outcomes and early intervention rates across the region.
U.K. Fetal Valproate Syndrome Market Insight
The U.K. Fetal Valproate Syndrome market is anticipated to grow significantly during the forecast period, driven by the government’s continued investigations into valproate use during pregnancy and national awareness campaigns led by patient advocacy groups. Increasing integration of fetal anomaly screening programs within the NHS and the development of multidisciplinary clinics for affected children are expected to improve diagnosis and support services, thereby propelling market expansion.
Germany Fetal Valproate Syndrome Market Insight
The Germany Fetal Valproate Syndrome market is expected to expand at a notable CAGR, backed by the country’s well-structured healthcare infrastructure, high adoption of genetic testing, and focus on preventive maternal health. German research institutions are actively contributing to studies assessing long-term developmental outcomes in children exposed to valproate, while local health authorities continue to strengthen regulations on prenatal drug use.
Asia-Pacific Fetal Valproate Syndrome Market Insight
The Asia-Pacific Fetal Valproate Syndrome market is projected to grow at the fastest CAGR of 24% during 2025–2032, driven by improving maternal healthcare systems, increasing access to prenatal diagnostics, and growing public awareness campaigns. Countries such as India, Japan, and China are witnessing rising healthcare investments and digitalization of maternal health records, facilitating early risk detection. The region’s expanding healthcare infrastructure and focus on reducing congenital disorders through education and policy initiatives are further propelling market growth.
Japan Fetal Valproate Syndrome Market Insight
The Japan Fetal Valproate Syndrome market is gaining momentum due to strong government support for maternal health programs and increased collaboration between hospitals and research organizations. The country’s advanced perinatal care system, emphasis on genetic screening, and awareness campaigns around antiepileptic drug safety are improving early diagnosis and intervention rates.
China Fetal Valproate Syndrome Market Insight
The China Fetal Valproate Syndrome market accounted for the largest revenue share in the Asia-Pacific region in 2024, attributed to expanding healthcare infrastructure, rising disposable income, and growing awareness of prenatal drug-related risks. The government’s investments in maternal and child health initiatives, combined with the growing availability of affordable diagnostic and treatment options, are expected to sustain market growth over the forecast period.
Fetal Valproate Syndrome Market Share
The Fetal Valproate Syndrome industry is primarily led by well-established companies, including:
• GSK. plc (U.K.)
• Pfizer Inc. (U.S.)
• Sanofi (France)
• Roche Holding AG (Switzerland)
• Novartis AG (Switzerland)
• AstraZeneca plc (U.K.)
• Lilly (U.S.)
• Bayer AG (Germany)
• Johnson & Johnson and its affiliates(U.S.)
• Teva Pharmaceutical Industries Ltd. (Israel)
• AbbVie Inc. (U.S.)
• Merck & Co., Inc. (U.S.)
• Takeda Pharmaceutical Company Limited (Japan)
• Viatris Inc. (U.S.)
• Boehringer Ingelheim International GmbH (Germany)
• Amgen Inc. (U.S.)
• Vertex Pharmaceuticals Incorporated (U.S.)
• Biogen Inc. (U.S.)
• Alexion Pharmaceuticals, Inc. (U.S.)
• Orphan Technologies Ltd. (Switzerland)
Latest Developments in Global Fetal Valproate Syndrome Market
- In November 2023, a cross-sectional study conducted across multiple European countries revealed that individuals diagnosed with Fetal Valproate Syndrome (FVS) showed high levels of cognitive and developmental impairments. The study reported that over 43% of patients experienced moderate cognitive disabilities, while 14% exhibited severe impairment. Furthermore, autism spectrum and sensory disorders were present in more than 60% of cases. This data highlighted the urgent need for advanced therapeutic approaches and better support systems, driving increased research and investment in early-intervention programs within the FVS treatment landscape
- In January 2024, the European Medicines Agency (EMA) implemented new precautionary measures concerning children born to men treated with valproate around conception. This regulatory action followed Nordic registry findings linking paternal valproate exposure with higher risks of neurodevelopmental disorders in offspring. The move is expected to shape global regulatory frameworks and encourage pharmaceutical manufacturers to enhance risk-management programs and patient-education initiatives related to valproate therapies
- In March 2024, a multinational observational study found that the declining use of sodium valproate among pregnant women directly correlated with a reduction in birth defects and neurodevelopmental abnormalities associated with Fetal Valproate Syndrome. These findings have encouraged healthcare providers to adopt stricter prescription controls, contributing to improved patient safety standards and a gradual decline in FVS incidence rates
- In June 2025, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) issued updated drug safety guidance, reinforcing that in-utero exposure to valproate poses significant risks, including an 11% chance of major birth defects and up to a 40% likelihood of neurodevelopmental disorders. The updated policy expanded pregnancy-prevention and patient-monitoring programs, prompting pharmaceutical companies to intensify their compliance and education strategies, thereby influencing market dynamics and safety-driven innovations in the FVS treatment domain
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.