Global Fraxiparine Market
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
2.53 Billion
2024
2032
USD
1.70 Billion
USD
2.53 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 2.53 Billion | |
|
|
|
|
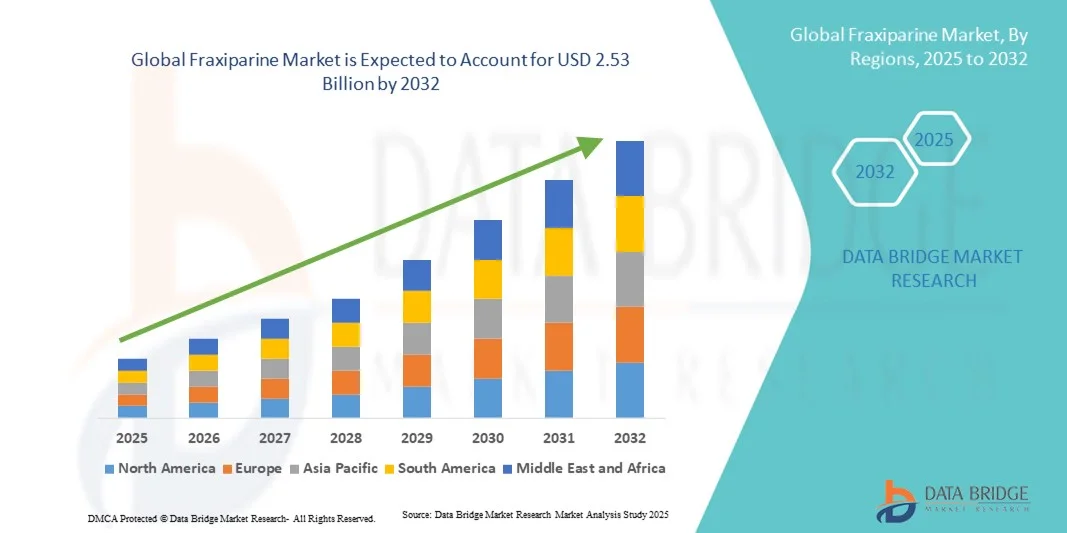
Fraxiparine Market Size
- The global fraxiparine market size was valued at USD 1.7 billion in 2024 and is expected to reach USD 2.53 billion by 2032, at a CAGR of 5.10% during the forecast period
- The market growth is largely fueled by the increasing prevalence of thromboembolic disorders and the rising use of anticoagulant therapies like Fraxiparine (nadroparin calcium) in both hospital and outpatient settings. Advances in clinical research, formulation improvements, and adoption of preventive therapies for conditions such as deep vein thrombosis (DVT) and pulmonary embolism (PE) are driving significant growth within the Fraxiparine market
- Furthermore, growing awareness among healthcare professionals and patients regarding the benefits of low-molecular-weight heparins, along with supportive government initiatives and expanding healthcare infrastructure, is accelerating the adoption of Fraxiparine solutions. These converging factors are establishing Fraxiparine as a preferred anticoagulant therapy, thereby significantly boosting the industry’s growth
Fraxiparine Market Analysis
- Fraxiparine, a low-molecular-weight heparin, is increasingly vital in the prevention and treatment of thromboembolic disorders such as deep vein thrombosis (DVT), pulmonary embolism (PE), and other cardiovascular conditions, due to its efficacy, predictable pharmacokinetics, and ease of use in both hospital and outpatient settings
- The escalating demand for fraxiparine is primarily fueled by the rising prevalence of cardiovascular and thromboembolic diseases, growing awareness among healthcare professionals and patients, and increasing adoption of preventive and therapeutic anticoagulant therapies
- North America dominated the fraxiparine market with the largest revenue share of 40.5% in 2024, driven by well-established healthcare infrastructure, high incidence of cardiovascular disorders, and strong presence of key industry players. The U.S. experienced substantial growth in Fraxiparine adoption, particularly in hospitals and specialty clinics, supported by advancements in anticoagulant therapies and clinical guidelines
- Asia-Pacific is expected to be the fastest-growing region in the fraxiparine market during the forecast period, with a projected CAGR due to increasing urbanization, rising disposable incomes, and improving healthcare infrastructure
- Adults dominated the market with a revenue share of 68% in 2024, as the prevalence of cardiovascular diseases, thromboembolic disorders, and post-operative complications is higher in this group. Hospital protocols widely favor Fraxiparine for adults undergoing surgeries or long-term anticoagulation therapy
Report Scope and Fraxiparine Market Segmentation
|
Attributes |
Fraxiparine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Fraxiparine Market Trends
Improved Therapeutic Efficacy and Patient-Centric Treatment
- A significant and accelerating trend in the global fraxiparine market is the increasing adoption of novel anticoagulant formulations and delivery systems designed to enhance treatment efficacy, patient safety, and compliance. These innovations are significantly improving therapeutic outcomes and offering clinicians more precise control over anticoagulation therapy
- For instance, the introduction of low-molecular-weight fraxiparine formulations with improved pharmacokinetic profiles allows for more predictable dosing and reduced risk of bleeding complications. Similarly, prefilled syringes and multi-dose delivery devices provide convenience and ensure accurate dosing for both in-hospital and at-home use
- Advancements in patient monitoring and clinical protocols enable healthcare providers to personalize Fraxiparine therapy based on individual risk profiles, such as renal function, body weight, and co-morbidities. For instance, novel dosing algorithms and mobile health platforms assist clinicians in monitoring anticoagulation levels and adjusting doses safely. Furthermore, extended-release formulations and combination therapies are being developed to enhance treatment adherence and clinical outcomes
- The integration of Fraxiparine therapy with broader clinical pathways, including perioperative care, thromboprophylaxis, and chronic disease management, facilitates centralized monitoring and optimized patient care. Through coordinated treatment plans, healthcare professionals can manage anticoagulation alongside other therapies, creating a comprehensive and efficient care experience
- This trend towards more precise, patient-centric, and clinically integrated Fraxiparine therapy is fundamentally reshaping treatment standards and patient expectations. Consequently, companies such as Sanofi and Aspen are developing new Fraxiparine formulations with enhanced stability, extended shelf-life, and optimized dosing schedules to improve clinical convenience
- The demand for advanced Fraxiparine solutions is growing rapidly across hospitals, specialty clinics, and home healthcare settings, as clinicians and patients increasingly prioritize efficacy, safety, and ease of administration
Fraxiparine Market Dynamics
Driver
Growing Need Due to Rising Incidence of Thromboembolic Disorders and Hospitalization
- The increasing prevalence of conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and post-surgical thromboembolic complications is a significant driver for the heightened demand for Fraxiparine
- For instance, in April 2024, Sanofi announced the launch of an enhanced Fraxiparine prefilled syringe formulation for high-risk post-operative patients, enabling simplified dosing and safer administration in hospital settings. Such strategies by key companies are expected to drive the Fraxiparine industry growth in the forecast period
- As patients and healthcare providers become more aware of thromboembolic risks and the importance of early intervention, Fraxiparine offers advantages such as predictable anticoagulant response, reduced monitoring requirements, and lower risk of adverse events compared to traditional anticoagulants
- Furthermore, the growing prevalence of chronic conditions requiring anticoagulation therapy, combined with an increase in surgical procedures, is expanding the need for reliable Fraxiparine treatment across healthcare facilities
- The ease of administration, suitability for both inpatient and outpatient care, and ability to manage high-risk patient populations are key factors propelling the adoption of Fraxiparine. Increasing awareness programs and clinician education on dosing and safety protocols further contribute to market growth
Restraint/Challenge
Concerns Regarding Adverse Effects and High Treatment Costs
- Concerns surrounding the risk of bleeding, heparin-induced thrombocytopenia (HIT), and other adverse effects pose a significant challenge to broader market adoption. As Fraxiparine therapy requires careful patient selection and monitoring, clinicians may be cautious in prescribing it for certain populations
- For instance, reports of dose-related bleeding complications have made some healthcare providers hesitant to use Fraxiparine in complex cases without stringent monitoring protocols
- Addressing these concerns through clinical education, patient monitoring tools, and clear dosing guidelines is crucial for building confidence among healthcare professionals. Companies such as Sanofi emphasize safety protocols, pharmacovigilance, and clear administration instructions in their marketing to reassure potential prescribers
- In addition, the relatively high cost of Fraxiparine compared to generic anticoagulants can be a barrier for price-sensitive healthcare systems or patients in emerging markets
- While prices have gradually decreased in some regions, the perception of a premium for advanced formulations can still hinder widespread adoption, especially where generic alternatives are available
- Overcoming these challenges through cost optimization, enhanced safety measures, and broader clinician awareness programs will be vital for sustained growth in the Fraxiparine market
Fraxiparine Market Scope
The market is segmented on the basis of indication, population type, dosage strength, application, end-user, and distribution channel.
- By Indication
On the basis of indication, the Fraxiparine market is segmented into Angina, Myocardial Infarction, Thromboembolism, and Thrombosis. Thromboembolism dominated the market with a revenue share of 44% in 2024, driven by the rising prevalence of deep vein thrombosis (DVT), pulmonary embolism (PE), and related vascular disorders globally. The segment benefits from increasing awareness among physicians and patients about early diagnosis and prophylactic treatments. Hospitals and clinics prefer Fraxiparine for thromboembolism due to its predictable anticoagulant effect and lower risk of heparin-induced thrombocytopenia. The growing geriatric population, coupled with increasing post-surgery thromboembolic risks, further fuels demand. Expansion of healthcare infrastructure and the inclusion of Fraxiparine in standard treatment protocols for thromboembolic conditions reinforce its dominant market position. Moreover, the introduction of pre-filled syringes and easier administration options enhances its adoption across hospital settings. Reimbursement policies in developed countries also support the high uptake of Fraxiparine in thromboembolism treatment. Clinical studies highlighting efficacy and safety profiles strengthen physician confidence. Awareness campaigns and collaborations with hematology societies encourage broader utilization. The segment’s dominance is also aided by hospital guidelines that prioritize low molecular weight heparins for thromboembolic risk management. In addition, high patient compliance due to convenient dosing schedules contributes to sustained revenue growth in this subsegment.
Thrombosis is expected to witness the fastest CAGR of 7.2% from 2025 to 2032, driven by rising cases of post-surgical thrombosis, increasing prevalence of obesity, and expanding cardiovascular disease burden worldwide. Emerging markets with improving healthcare infrastructure are adopting Fraxiparine in thrombosis management. Rising awareness among clinicians regarding early intervention and prophylactic therapy further propels growth. Technological advancements in syringe formulations and precise dosing systems support increased usage. Educational programs for healthcare professionals on thrombosis risk management and treatment protocols also contribute. Growing outpatient surgical procedures and homecare anticoagulation therapy expand the target patient base. Clinical trials demonstrating superior efficacy over other anticoagulants enhance physician trust. Favorable reimbursement frameworks in several countries encourage adoption. The segment benefits from increasing collaborations between pharmaceutical companies and hospitals for product training. Patient preference for minimally invasive administration methods boosts demand. Strategic marketing and distribution initiatives further accelerate penetration. Overall, improved diagnosis, prevention strategies, and clinical adoption drive the fastest growth in this segment.
- By Population Type
On the basis of population type, the Fraxiparine market is segmented into Children and Adults.
Adults dominated the market with a revenue share of 68% in 2024, as the prevalence of cardiovascular diseases, thromboembolic disorders, and post-operative complications is higher in this group. Hospital protocols widely favor Fraxiparine for adults undergoing surgeries or long-term anticoagulation therapy. The segment benefits from extensive clinical trials supporting efficacy and safety in adult populations. Adults constitute the largest pool of post-surgical patients requiring anticoagulation. High physician awareness and adherence to clinical guidelines drive adoption. The availability of multiple dosage strengths enhances flexibility in therapy. Insurance coverage and reimbursement policies in developed countries contribute to market stability. The convenience of pre-filled syringes increases patient compliance. Growing outpatient procedures also support adoption. Pharmaceutical companies prioritize adult patient education programs, enhancing brand loyalty. Hospitals stock Fraxiparine extensively for adult care. The segment’s dominance is reinforced by evidence-based treatment protocols. Awareness campaigns by hematology societies further support usage. Overall, the adult population continues to drive majority market revenue due to high demand and consistent clinical adoption.
Children are expected to witness the fastest CAGR of 6.5% from 2025 to 2032, supported by increasing awareness of pediatric thromboembolic risks, improved diagnosis of congenital heart defects, and safer anticoagulation protocols. Pediatric-specific dosing syringes and training programs for clinicians accelerate growth. Hospitals and specialty clinics increasingly adopt Fraxiparine for neonatal and pediatric care. Clinical studies validating safety in children enhance adoption. Educational initiatives for caregivers further support usage. Government healthcare programs targeting pediatric care expand access. Growth is also driven by improved monitoring tools and minimally invasive administration. The rise in pediatric surgeries requiring anticoagulation contributes to demand. Collaborations between pediatric hospitals and pharmaceutical companies strengthen penetration. Parent awareness campaigns emphasize early treatment, encouraging therapy initiation. Regional healthcare expansions in Asia-Pacific and Latin America boost the segment. Overall, pediatric adoption grows rapidly due to clinical advancements and rising awareness.
- By Dosage Strength
On the basis of dosage strength, the Fraxiparine market is segmented into 0.2 mL (1,900 Anti-Xa IU), 0.3 mL (2,850 Anti-Xa IU), 0.4 mL (3,800 Anti-Xa IU), 0.6 mL (5,700 Anti-Xa IU), 1.0 mL (9,500 Anti-Xa IU), 0.6 mL (11,400 Anti-Xa IU), 0.8 mL (15,200 Anti-Xa IU), and 1.0 mL (19,000 Anti-Xa IU). The 0.6 mL (5,700 Anti-Xa IU) dosage dominated the market with a revenue share of 42% in 2024, owing to its widespread adoption in standard prophylactic and therapeutic protocols. Hospitals prefer this strength for general adult treatments due to its efficacy and flexible dosing. The dosage is suitable for post-surgical thromboprophylaxis, outpatient anticoagulation, and routine clinical use. Physician familiarity with this formulation ensures consistent prescribing patterns. Pre-filled syringes reduce dosing errors and improve patient compliance. The availability across multiple regions ensures steady supply and adoption. Clinical trials demonstrate consistent anticoagulation effect, reinforcing confidence. The formulation aligns with hospital inventory protocols and patient convenience requirements. Regulatory approvals in key markets further support its dominance. The segment benefits from widespread insurance coverage and reimbursement. Education and training for clinicians improve handling and administration. Growth in elective surgeries also drives usage. The segment remains the backbone of Fraxiparine therapy globally.
The 1.0 mL (19,000 Anti-Xa IU) dosage is expected to witness the fastest CAGR of 7.8% from 2025 to 2032, driven by demand for high-dose treatments in obese and high-risk patients. Specialized clinical protocols require higher doses for effective anticoagulation, fueling adoption. Expansion of surgical procedures and inpatient care in emerging markets increases usage. Hospitals and specialty clinics are incorporating high-dose syringes to reduce dosing errors. Physician awareness campaigns and training programs promote correct use. Technological improvements in syringe design ensure safety and precision. Clinical studies highlight its efficacy in severe cases and complex thrombotic conditions. Insurance coverage is expanding to support high-risk patient treatment. Growth is particularly strong in developed regions with aging populations. Increasing elective orthopedic and cardiac surgeries requiring high doses contribute to market expansion. Awareness among hematologists and cardiologists accelerates adoption. Manufacturers focus on strategic distribution and hospital partnerships. Overall, this dosage strength captures the fastest growth due to clinical necessity and demographic trends.
- By Application
On the basis of application, the Fraxiparine market is segmented into General Surgery, Orthopaedic Surgery, and Others. General Surgery dominated the market with a revenue share of 46% in 2024, driven by the high risk of post-operative thromboembolic events and the integration of Fraxiparine into surgical prophylaxis protocols. The segment benefits from standardized hospital procedures recommending low molecular weight heparins. Surgeons prefer Fraxiparine due to its predictable pharmacokinetics and low risk of complications. Broad hospital adoption and multi-region regulatory approvals reinforce dominance. Pre-filled syringe formulations improve safety and reduce dosing errors. Insurance and reimbursement frameworks in key markets ensure accessibility. Hospitals stock the product extensively for inpatient and day-surgery procedures. Physician confidence is supported by numerous clinical trials. Post-surgical guidelines encourage use for high-risk patient populations. The segment sees steady demand due to routine surgeries and increasing surgical volumes globally. Continuous professional training and hospital partnerships enhance utilization. Patient compliance is improved due to convenient administration. Overall, general surgery remains the primary revenue contributor.
Orthopaedic Surgery is expected to witness the fastest CAGR of 7.5% from 2025 to 2032, driven by the growing number of joint replacement surgeries, trauma interventions, and aging populations requiring thromboprophylaxis. Hospitals increasingly adopt Fraxiparine in elective and emergency orthopedic procedures. Surgeons prefer standardized anticoagulation protocols to minimize complications. Pre-filled syringes enhance dosing accuracy and reduce errors in high-risk procedures. Regional healthcare expansions in Asia-Pacific and Latin America contribute to demand. Educational campaigns targeting orthopedic surgeons encourage adoption. Clinical studies demonstrate efficacy in preventing post-operative thromboembolic events. Insurance coverage is expanding to support orthopedic care. Outpatient orthopedic surgeries and fast-track recovery programs increase the target patient pool. Manufacturer initiatives and hospital collaborations improve penetration. Rising awareness of post-surgical thrombosis risk accelerates adoption. Overall, orthopaedic surgery represents the fastest-growing application segment.
- By End-User
On the basis of end-user, the Fraxiparine market is segmented into Hospitals, Specialty Clinics, and Others. Hospitals dominated the market with a revenue share of 61% in 2024, driven by the high volume of surgical procedures, inpatient care, and post-operative anticoagulation therapy. Hospitals stock Fraxiparine extensively due to standardized protocols recommending its use for thromboembolic prevention. Clinical trials supporting safety and efficacy in high-risk patients reinforce physician preference. Pre-filled syringe formulations enhance dosing accuracy and reduce administration errors. Regulatory approvals in major markets ensure uninterrupted supply and adoption. Hospitals benefit from predictable inventory and high patient throughput. Reimbursement frameworks in developed countries facilitate wide accessibility. Physicians rely on Fraxiparine for both adult and geriatric populations. Continuous hospital staff training promotes proper usage. The segment is supported by partnerships between manufacturers and hospital pharmacy departments. Awareness campaigns among healthcare professionals boost adoption. Consistent prescribing patterns and post-surgical guidelines maintain strong demand. Overall, hospitals remain the largest end-user segment due to operational scale and clinical protocols.
Specialty Clinics are expected to witness the fastest CAGR of 6.9% from 2025 to 2032, fueled by the growing number of outpatient procedures, increased focus on preventive care, and rising adoption of specialized anticoagulation services. Clinics treating cardiovascular, orthopedic, and hematological conditions increasingly rely on Fraxiparine. Patient convenience and pre-filled syringe formats support rapid adoption. Regional healthcare expansions in emerging markets contribute to growth. Physician awareness programs in specialty fields enhance trust and usage. Clinics are incorporating Fraxiparine into standard therapy for thromboembolic risk management. Education of clinical staff ensures accurate dosing and monitoring. Expanding insurance coverage for outpatient care drives uptake. Collaborative programs between pharmaceutical companies and clinics improve access. The segment benefits from rising elective surgeries conducted in specialty clinics. Patient demand for convenient and effective anticoagulation therapy increases adoption. Digital prescription and e-pharmacy support further enhance reach. Overall, specialty clinics represent the fastest-growing end-user segment.
- By Distribution Channel
On the basis of distribution channel, the Fraxiparine market is segmented into Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others. Hospital Pharmacies dominated the market with a revenue share of 58% in 2024, as Fraxiparine is primarily administered in clinical settings under physician supervision. Direct procurement through hospital pharmacies ensures supply reliability and adherence to treatment protocols. Hospitals prioritize stocking pre-filled syringes and high-demand dosages. Regulatory approvals and clinical guidelines reinforce the channel’s dominance. Hospital pharmacy distribution facilitates patient monitoring and dosage accuracy. Partnerships with manufacturers ensure consistent availability. High patient volumes and post-surgical procedures support steady revenue. Hospitals provide training to staff for safe administration. Insurance and reimbursement coverage further support uptake. Inventory management and bulk procurement reduce costs. Clinical adoption of Fraxiparine for adults and geriatric patients strengthens this channel. Overall, hospital pharmacies remain the leading distribution channel due to operational efficiency and direct patient access.
Online Pharmacies are expected to witness the fastest CAGR of 8.1% from 2025 to 2032, driven by the increasing trend of home delivery, convenience for patients under long-term anticoagulation therapy, and the expansion of e-pharmacy platforms globally. Digital ordering simplifies access for patients in remote areas. Patient preference for doorstep delivery and subscription models accelerates growth. Online pharmacies provide access to multiple dosage strengths and formulations. Regional expansion in Asia-Pacific and Latin America supports penetration. Awareness campaigns and telemedicine integration enhance adoption. Manufacturers are collaborating with e-pharmacies for promotions and training. Insurance coverage for home delivery improves affordability. Convenience, combined with secure packaging, ensures patient compliance. E-pharmacies facilitate automated refill services and dosage reminders. Educational content for patients boosts trust and safe usage. Overall, online pharmacies represent the fastest-growing distribution channel segment due to convenience and digital adoption.
Fraxiparine Market Regional Analysis
- North America dominated the fraxiparine market with the largest revenue share of 40.5% in 2024, driven by the presence of advanced healthcare infrastructure, ongoing research in anticoagulation therapies, and high prevalence of thromboembolic disorders
- Healthcare providers in the region highly value the efficacy, safety, and predictability offered by Fraxiparine, making it a preferred choice for both hospital-based and outpatient anticoagulation management
- This widespread adoption is further supported by high healthcare expenditure, advanced clinical capabilities, and a strong focus on personalized patient care, establishing Fraxiparine as a leading anticoagulant therapy across North America
U.S. Fraxiparine Market Insight
The U.S. fraxiparine market captured the largest revenue share in 2024 within North America, fueled by rapid advancements in low-molecular-weight heparin formulations, increasing prevalence of DVT and pulmonary embolism, and rising adoption of hospital-based anticoagulation protocols. The growing emphasis on patient safety, clinical efficacy, and streamlined dosing regimens further propels the Fraxiparine industry. Moreover, ongoing investments in research and development for improved therapeutic options are significantly contributing to market expansion.
Europe Fraxiparine Market Insight
The Europe fraxiparine market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising incidence of thromboembolic disorders, stringent healthcare regulations, and increasing focus on evidence-based anticoagulation therapy. Growing urbanization and enhanced clinical infrastructure are supporting the adoption of Fraxiparine in hospitals and specialty clinics. European healthcare providers are increasingly leveraging Fraxiparine for perioperative and long-term anticoagulation management, contributing to strong market growth across the region.
U.K. Fraxiparine Market Insight
The U.K. fraxiparine market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of thromboembolic risks, enhanced hospital protocols, and increasing adoption of standardized anticoagulation treatment. Additionally, government initiatives promoting patient safety and improved access to anticoagulants are encouraging hospitals and specialty clinics to adopt Fraxiparine. The country’s robust healthcare infrastructure and well-established clinical guidelines are expected to continue driving market growth.
Germany Fraxiparine Market Insight
The Germany fraxiparine market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing prevalence of cardiovascular and thrombotic disorders, emphasis on advanced clinical care, and growing adoption of evidence-based anticoagulation therapies. Germany’s well-developed hospital and clinical infrastructure, combined with its focus on innovation and safety, supports widespread use of Fraxiparine. Additionally, the integration of Fraxiparine into perioperative and long-term treatment protocols is enhancing patient outcomes and driving demand.
Asia-Pacific Fraxiparine Market Insight
The Asia-Pacific fraxiparine market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by increasing incidence of thromboembolic disorders, rising healthcare expenditure, and expansion of hospital and specialty clinic networks in countries such as China, Japan, and India. The region's growing focus on improving clinical outcomes and expanding access to anticoagulation therapy is supporting rapid Fraxiparine adoption. Furthermore, the availability of cost-effective formulations and government healthcare initiatives is expanding accessibility across both urban and semi-urban populations.
Japan Fraxiparine Market Insight
The Japan fraxiparine market is gaining momentum due to the country’s advanced healthcare system, increasing awareness of thromboembolic risks, and rising adoption of evidence-based anticoagulation protocols. Japanese hospitals and specialty clinics are increasingly using Fraxiparine for both surgical prophylaxis and long-term management of high-risk patients. Additionally, the aging population and emphasis on patient safety are expected to further drive demand in residential and clinical care settings.
China Fraxiparine Market Insight
The China fraxiparine market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the country’s expanding healthcare infrastructure, growing number of hospitals and specialty clinics, rising prevalence of thromboembolic and cardiovascular disorders, and increasing public awareness of anticoagulation therapy. The availability of cost-effective Fraxiparine options, combined with strong local pharmaceutical manufacturing, is supporting widespread adoption across urban and semi-urban healthcare settings.
Fraxiparine Market Share
The Fraxiparine industry is primarily led by well-established companies, including:
- Sanofi (France)
- Pfizer Inc. (U.S.)
- Boehringer Ingelheim GmbH (Germany)
- GSK plc (U.K.)
- Bayer AG (Germany)
- Abbott (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Eisai Co., Ltd. (Japan)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Aspen Pharmacare (South Africa)
- Roche Holding AG (Switzerland)
- Amgen Inc. (U.S.)
- Novartis AG (Switzerland)
Latest Developments in Global Fraxiparine Market
- In May 2021, Chiesi Global Rare Diseases announced the FDA approval of FERRIPROX® (deferiprone) for the treatment of transfusional iron overload in patients with thalassemia syndromes. This approval underscores Chiesi's commitment to expanding its rare disease portfolio and providing innovative therapies to address unmet medical needs in hematology
- In August 2023, PRNewswire reported that the global antithrombotic drugs market size is estimated to increase by USD 23,199.94 million from 2022 to 2027, driven by the growing prevalence of coagulation disorders. This market growth highlights the increasing demand for effective anticoagulant therapies, including Fraxiparine, to manage thromboembolic condition
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.