Global Freeze Dried Vaccine Kit Market
Market Size in USD Billion
CAGR :
% 
 USD
11.29 Billion
USD
26.61 Billion
2024
2032
USD
11.29 Billion
USD
26.61 Billion
2024
2032
| 2025 –2032 | |
| USD 11.29 Billion | |
| USD 26.61 Billion | |
|
|
|
|
Freeze-Dried Vaccine Kit Market Size
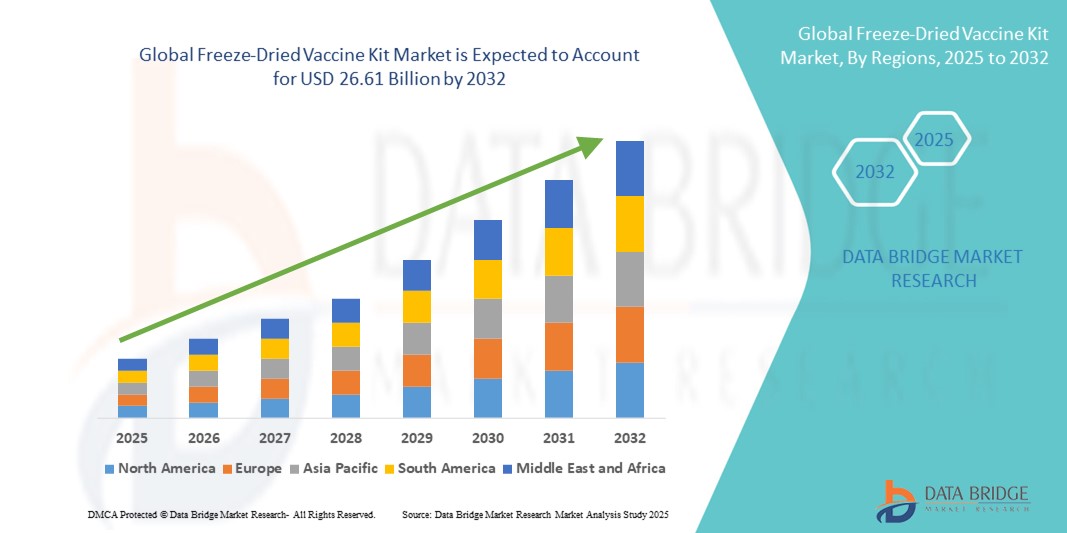
- The global freeze-dried vaccine kit market size was valued at USD 11.29 billion in 2024 and is expected to reach USD 26.61 billion by 2032, at a CAGR of 11.31% during the forecast period
- The market growth is largely driven by increasing demand for long-shelf-life vaccines that can be stored and transported without continuous refrigeration, particularly in low-resource settings and emerging economies
- Furthermore, the rising prevalence of infectious diseases, expanding immunization programs, and government-led vaccination initiatives are contributing to the growing adoption of freeze-dried vaccine kits across healthcare systems. These combined forces are propelling the expansion of the freeze-dried vaccine kit market, reinforcing its role in global public health infrastructure
Freeze-Dried Vaccine Kit Market Analysis
- Freeze-dried vaccine kits, which include lyophilized vaccines along with reconstitution components, are increasingly essential in global immunization efforts due to their extended shelf life, temperature stability, and suitability for distribution in remote or low-resource environments where cold chain maintenance is challenging
- The accelerating demand for freeze-dried vaccine kits is primarily driven by rising global vaccination campaigns, growing incidence of infectious diseases, and increasing reliance on stable vaccine formulations that ensure efficacy during long-distance transportation and storage
- North America dominated the freeze-dried vaccine kit market with the largest revenue share of 39.2% in 2024, supported by strong healthcare infrastructure, early adoption of advanced vaccine technologies, and well-established immunization programs, particularly in the U.S., which leads in procurement and deployment of lyophilized vaccines across both public and private sectors
- Asia-Pacific is expected to be the fastest growing region in the freeze-dried vaccine kit market during the forecast period, fueled by large-scale immunization drives, expanding vaccine manufacturing capacities in countries such as India and China, and increasing investments in public health infrastructure
- The rabies vaccine segment dominated the freeze-dried vaccine kit market with a market share of 45.8% in 2024, driven by its critical use in both pre- and post-exposure prophylaxis and strong demand in regions with high rabies prevalence, supported by WHO guidelines and government-backed vaccination programs
Report Scope and Freeze-Dried Vaccine Kit Market Segmentation
|
Attributes |
Freeze-Dried Vaccine Kit Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Freeze-Dried Vaccine Kit Market Trends
Rising Demand for Temperature-Stable Vaccines in Global Immunization Programs
- A significant and accelerating trend in the global freeze-dried vaccine kit market is the increasing demand for lyophilized vaccines that offer improved temperature stability and extended shelf life, particularly in regions with limited cold chain infrastructure. This trend is driven by the need to ensure reliable immunization in remote and underserved areas across Asia, Africa, and Latin America
- For instance, the World Health Organization (WHO) and Gavi, the Vaccine Alliance, have prioritized freeze-dried vaccines such as rabies and BCG in their global immunization campaigns due to their ability to remain stable without continuous refrigeration. Manufacturers such as Serum Institute of India and Sanofi are scaling up production of such kits to meet rising demand
- Innovations in packaging, such as dual-chamber syringes and integrated reconstitution systems, are also enhancing usability in field conditions by reducing administration complexity and the risk of contamination. This makes freeze-dried vaccine kits more accessible and reliable in mass immunization programs
- These developments support efficient storage, transportation, and deployment of vaccines in emergency settings, making freeze-dried kits a strategic component of pandemic preparedness and disease outbreak response
- As public health agencies and global organizations increase investments in resilient vaccine delivery systems, the adoption of freeze-dried vaccine kits is expected to accelerate, transforming them into a standard choice for global immunization efforts
- The growing emphasis on accessibility, logistical efficiency, and long-term vaccine stability is reshaping procurement strategies and fueling innovation in the freeze-dried vaccine kit landscape
Freeze-Dried Vaccine Kit Market Dynamics
Driver
Surge in Infectious Disease Burden and Global Immunization Initiatives
- The increasing prevalence of infectious diseases and renewed global focus on universal immunization are major drivers of the freeze-dried vaccine kit market. Governments and health organizations are expanding routine immunization programs, creating a sustained demand for temperature-stable, easy-to-distribute vaccine solutions
- For instance, national health agencies across Asia-Pacific and Africa are incorporating freeze-dried rabies and BCG vaccines into their public immunization schedules to combat preventable diseases and improve access in remote regions
- Freeze-dried vaccine kits offer a distinct advantage in terms of transport and storage, allowing for deployment in areas lacking advanced refrigeration systems. Their ability to maintain potency under varying conditions makes them essential during public health emergencies, outbreaks, and humanitarian crises
- Growing collaborations between international agencies, governments, and vaccine manufacturers are leading to large-scale procurement and distribution of freeze-dried kits, especially in countries with high disease burdens
- In addition, increased awareness of pandemic preparedness and the need for scalable, shelf-stable vaccines is further boosting investment in freeze-dried vaccine technologies across both public and private sectors
Restraint/Challenge
Regulatory Complexity and Operational Limitations in Low-Resource Settings
- Despite their advantages, freeze-dried vaccine kits face challenges related to regulatory complexity and on-ground administration. Differing approval processes across countries can slow market entry and distribution, especially in regions with stringent stability and packaging requirements
- For instance, manufacturers must navigate varying international standards to supply to agencies such as UNICEF or local governments, which can increase compliance costs and delay access
- In low-resource settings, the need for reconstitution before administration can pose a barrier due to limited healthcare staff training, lack of sterile environments, or difficulty managing diluent storage and handling
- In addition, some healthcare providers may prefer ready-to-use liquid vaccines due to familiarity, especially where infrastructure for training and supervision is weak. This can hinder the full adoption of freeze-dried kits without proper education and support systems
- Addressing these challenges requires streamlined regulatory frameworks, capacity-building initiatives, and investment in healthcare training to ensure proper use and widespread acceptance of freeze-dried vaccine kits in global health programs
Freeze-Dried Vaccine Kit Market Scope
The market is segmented on the basis of vaccine type, kit format, application, end user, and distribution channel.
- By Vaccine Type
On the basis of vaccine type, the freeze-dried vaccine kit market is segmented into rabies vaccine, BCG vaccine, yellow fever vaccine, typhoid vaccine, and other freeze-dried vaccines. The rabies vaccine segment dominated the market with the largest market revenue share of 45.8% in 2024, driven by its critical role in post-exposure prophylaxis and widespread use across regions with high rabies prevalence, particularly in Asia and Africa. Government-led mass vaccination programs and global health initiatives have increased the demand for rabies freeze-dried vaccine kits due to their extended shelf life and ease of transport.
The yellow fever vaccine segment is anticipated to witness the fastest growth rate of 7.9% from 2025 to 2032, fueled by expanding immunization coverage in Sub-Saharan Africa and Latin America. The need for reliable vaccine formulations in high-temperature regions and WHO-backed immunization efforts are contributing to increased uptake of freeze-dried yellow fever vaccines.
- By Kit Format
On the basis of kit format, the freeze-dried vaccine kit market is segmented into single-dose kit, multi-dose kit, and combination pack kit. The single-dose kit segment held the largest market revenue share in 2024, accounting for 51.8%, due to its advantages in infection control, simplified dosing, and reduced risk of contamination during mass immunization campaigns. These kits are particularly preferred in rural outreach and pediatric vaccination settings where safety and dosing accuracy are critical.
The combination pack kit segment is expected to witness the fastest CAGR of 8.3% from 2025 to 2032, driven by advancements in packaging design that enhance usability, storage efficiency, and time-saving administration in field conditions. These kits offer integrated components (vial, diluent, and syringe) and are increasingly used in emergency response situations.
- By Application
On the basis of application, the freeze-dried vaccine kit market is segmented into pre-exposure prophylaxis, post-exposure prophylaxis, tuberculosis immunization, and others. The post-exposure prophylaxis segment dominated the market with the largest revenue share of 47.1% in 2024, driven by widespread adoption of rabies vaccines in endemic regions, often distributed through government and NGO programs. The urgent need for rapid vaccine administration following potential exposure to viruses has made freeze-dried kits an essential stockpile product.
The tuberculosis immunization segment is anticipated to grow at the fastest rate of 7.5% from 2025 to 2032, supported by rising childhood immunization initiatives in high-burden countries. Freeze-dried BCG vaccines remain the primary intervention for TB prevention in newborns, contributing to consistent market demand.
- By End User
On the basis of end user, the freeze-dried vaccine kit market is segmented into hospitals, clinics, immunization centers, research institutes, and government organizations. The government organizations segment dominated the market with the largest revenue share of 42.6% in 2024, as national health ministries and international agencies such as UNICEF and WHO are the largest procurers of vaccine kits for public immunization programs. Their focus on reaching remote populations makes freeze-dried formats essential for logistical flexibility.
The immunization centers segment is expected to register the fastest growth rate of 8.1% from 2025 to 2032, driven by the expansion of dedicated vaccination facilities and increased public awareness about routine immunizations in both urban and rural regions.
- By Distribution Channel
On the basis of distribution channel, the freeze-dried vaccine kit market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, government supply chain, and wholesale distributors. The government supply chain segment held the largest market revenue share in 2024, accounting for 48.9%, due to centralized procurement and distribution through public health systems. These networks are supported by large-scale immunization funding from global health partnerships and donor agencies.
The online pharmacies segment is anticipated to witness the fastest growth rate of 9.2% from 2025 to 2032, fueled by digital transformation in healthcare and rising consumer interest in at-home vaccine services and remote prescription fulfillment. This trend is especially pronounced in urban markets with strong e-health infrastructure and regulatory support.
Freeze-Dried Vaccine Kit Market Regional Analysis
- North America dominated the freeze-dried vaccine kit market with the largest revenue share of 39.2% in 2024, supported by strong healthcare infrastructure, early adoption of advanced vaccine technologies, and well-established immunization programs, particularly in the U.S., which leads in procurement and deployment of lyophilized vaccines across both public and private sectors
- The U.S. plays a pivotal role with high adoption rates of freeze-dried vaccines in routine and travel immunization, along with a well-established supply chain for vaccine distribution across hospitals, clinics, and pharmacies
- Furthermore, the presence of major market players and continuous innovation in vaccine technology and packaging formats bolster the region’s leadership in the global freeze-dried vaccine kit market
U.S. Freeze-Dried Vaccine Kit Market Insight
The U.S. freeze-dried vaccine kit market captured the largest revenue share of 76.3% in 2024 within North America, driven by the country’s advanced healthcare infrastructure, high immunization coverage, and strong government vaccination programs. The presence of key pharmaceutical manufacturers and the widespread use of freeze-dried vaccines in national immunization schedules significantly contribute to market growth. In addition, increased travel vaccination demand, improved cold chain systems, and public-private partnerships for pandemic preparedness continue to fuel adoption across both public health and private healthcare facilities.
Europe Freeze-Dried Vaccine Kit Market Insight
The Europe freeze-dried vaccine kit market is projected to grow at a steady CAGR during the forecast period, supported by extensive routine immunization initiatives, well-established healthcare systems, and rising demand for long-shelf-life vaccine solutions. Regional emphasis on disease eradication programs and increasing public awareness regarding vaccination benefits are propelling the uptake of freeze-dried vaccines. The region is also experiencing heightened demand for travel and occupational vaccines, with government-supported procurement channels strengthening market accessibility.
U.K. Freeze-Dried Vaccine Kit Market Insight
The U.K. freeze-dried vaccine kit market is expected to witness notable growth due to consistent governmental support for immunization programs and high vaccine awareness among the population. The National Health Service (NHS) plays a critical role in distributing freeze-dried vaccines for diseases such as BCG and typhoid, especially in pediatric and traveler populations. Moreover, increasing imports of WHO-prequalified vaccines and public sector contracts with global vaccine manufacturers bolster the country’s contribution to the overall European market.
Germany Freeze-Dried Vaccine Kit Market Insight
The Germany freeze-dried vaccine kit market is poised for significant growth, driven by rising vaccination demand across pediatric and adult populations, particularly for tuberculosis and rabies. The country’s focus on biosecurity, health preparedness, and technological innovation in vaccine packaging supports the adoption of freeze-dried formats. In addition, Germany’s export-oriented pharmaceutical sector and research-driven approach facilitate early adoption of new immunization products and contribute to regional market expansion.
Asia-Pacific Freeze-Dried Vaccine Kit Market Insight
The Asia-Pacific freeze-dried vaccine kit market is anticipated to grow at the fastest CAGR of 22.5% during the forecast period of 2025 to 2032, owing to increasing public health awareness, large-scale immunization drives, and government funding in developing economies. Countries such as China, India, and Indonesia are focusing on improving vaccine cold chain logistics and enhancing access to rural populations, further boosting demand for freeze-dried formats due to their improved stability. The emergence of regional manufacturers and increasing support from international organizations are also key growth drivers.
Japan Freeze-Dried Vaccine Kit Market Insight
The Japan freeze-dried vaccine kit market is growing steadily, supported by a highly organized public health system, extensive adult vaccination programs, and a cultural focus on disease prevention. The country imports and manufactures a range of freeze-dried vaccines, particularly for tuberculosis and rabies. Increased international travel and preparations for health emergencies continue to drive the demand for freeze-dried kits due to their portability and long shelf life.
India Freeze-Dried Vaccine Kit Market Insight
The India freeze-dried vaccine kit market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to its expansive immunization programs such as Mission Indradhanush and growing private sector participation. India is both a leading consumer and exporter of freeze-dried vaccines, supported by a robust pharmaceutical manufacturing base and favorable regulatory frameworks. The country’s focus on expanding vaccine access in rural and underserved regions, alongside international partnerships for disease eradication, fuels sustained demand across public and private healthcare segments.
Freeze-Dried Vaccine Kit Market Share
The freeze-dried vaccine kit industry is primarily led by well-established companies, including:
- Sanofi (France)
- GSK (U.K.)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Serum Institute of India Pvt. Ltd. (India)
- Bio Farma (Indonesia)
- Bharat Biotech International Limited (India)
- Emergent BioSolutions Inc. (U.S.)
- Sinovac Biotech Ltd. (China)
- CSL Limited (Australia)
- Chengdu Institute of Biological Products Co., Ltd. (China)
- Moderna, Inc. (U.S.)
- AstraZeneca (U.K.)
- Indian Immunologicals Limited (India)
- Biological E. Limited (India)
- Zydus Lifesciences Limited (India)
- Meiji Seika Pharma Co., Ltd. (Japan)
- Panacea Biotec Limited (India)
- IDT Biologika GmbH (Germany)
- Hualan Biological Engineering Inc. (China)
What are the Recent Developments in Global Freeze-Dried Vaccine Kit Market?
- In April 2023, Serum Institute of India, one of the world’s largest vaccine manufacturers, expanded its production capabilities to include new freeze-dried vaccine lines aimed at improving vaccine shelf life and easing cold chain logistics across low-resource settings. This strategic move underscores the company’s commitment to advancing global immunization coverage, especially in regions where maintaining stringent refrigeration is challenging. The initiative also enhances Serum Institute’s footprint in the international vaccine supply market, strengthening its role in pandemic preparedness
- In March 2023, Sanofi announced a collaboration with UNICEF and Gavi to supply millions of doses of freeze-dried vaccines for measles and yellow fever across sub-Saharan Africa. This partnership supports the expansion of routine immunization programs and rapid outbreak response efforts. By leveraging freeze-dried formulations that remain stable at higher temperatures, the initiative addresses critical storage challenges and improves vaccine accessibility in remote regions, furthering Sanofi’s global public health impact
- In March 2023, Bio Farma, Indonesia’s state-owned pharmaceutical firm, inaugurated a high-capacity freeze-dried vaccine manufacturing facility with a focus on pediatric vaccines such as BCG and MR (measles-rubella). The investment aims to support the nation’s growing immunization needs and promote vaccine self-reliance. This development highlights Southeast Asia’s rising role in global vaccine supply chains and strengthens regional infrastructure for emergency and routine vaccine delivery
- In February 2023, GlaxoSmithKline (GSK) introduced a next-generation freeze-dried rabies vaccine with extended shelf life and rapid reconstitution features, specifically designed for use in resource-limited regions. The innovation aligns with global efforts to reduce rabies-related mortality and simplifies distribution in areas with unreliable cold chains. This launch emphasizes GSK’s focus on product innovation to improve vaccine reach and reliability worldwide
- In January 2023, UNICEF launched a global tender for the procurement of freeze-dried vaccine kits aimed at supporting outbreak response and humanitarian immunization efforts. The tender attracted leading global manufacturers and is part of a broader strategy to ensure timely availability of temperature-stable vaccines in emergencies. The move reflects increasing institutional demand for freeze-dried formats and reinforces their importance in strengthening global vaccine delivery systems
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.