Global Gammaretroviral Vector Market
Market Size in USD Billion
CAGR :
% 
 USD
1.23 Billion
USD
4.53 Billion
2024
2032
USD
1.23 Billion
USD
4.53 Billion
2024
2032
| 2025 –2032 | |
| USD 1.23 Billion | |
| USD 4.53 Billion | |
|
|
|
|
Gammaretroviral Vector Market Size
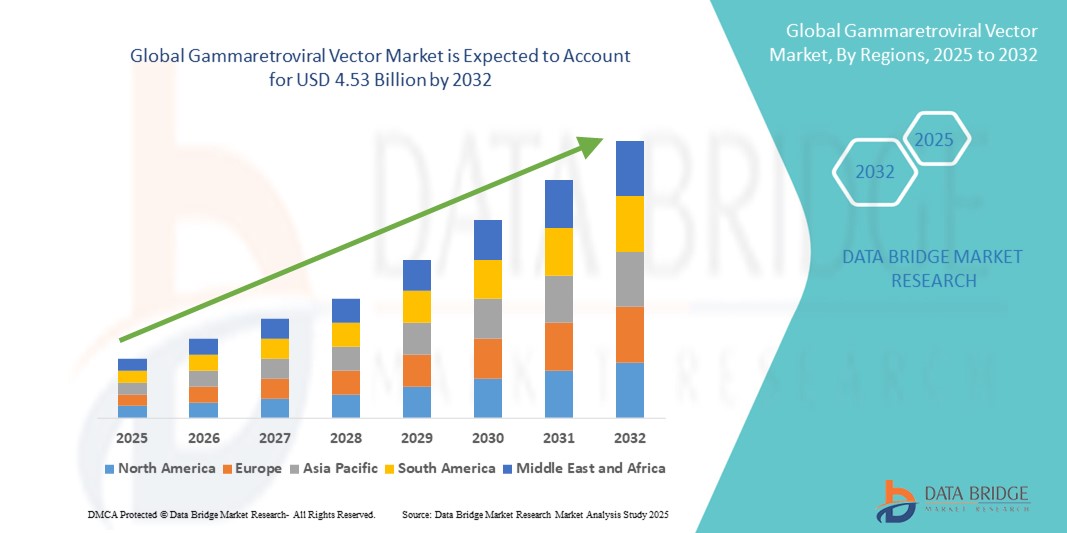
- The global gammaretroviral vector market size was valued at USD 1.23 billion in 2024 and is expected to reach USD 4.53 billion by 2032, at a CAGR of 17.7% during the forecast period
- The market growth is largely fueled by increasing research and clinical applications of gene therapy and genetic engineering, which have expanded the adoption of gammaretroviral vectors across various therapeutic areas
- Furthermore, rising demand for efficient, stable, and safe viral vectors for delivering therapeutic genes is driving the growth of the gammaretroviral vector market. These vectors are being widely utilized in ex vivo and in vivo gene therapies, as well as in cell-based therapies such as CAR-T cell treatments, thereby significantly boosting the industry's expansion
Gammaretroviral Vector Market Analysis
- The Gammaretroviral Vector market is witnessing significant growth globally, driven by the rising adoption of gene therapy, increasing investment in advanced biopharmaceutical research, and the expanding prevalence of genetic disorders
- The market benefits from technological advancements in viral vector design, enhanced safety profiles, and the growing number of clinical trials leveraging gammaretroviral vectors for treating rare and inherited diseases. Increasing government support for regenerative medicine and favorable reimbursement policies further bolster market expansion, particularly in North America and Europe
- North America dominated the gammaretroviral vector market with the largest revenue share of 41.5% in 2024, driven by well-established biotechnology infrastructure, high R&D investments, and the presence of leading pharmaceutical and gene therapy companies. The U.S. remains a major contributor, supported by increasing clinical trials, advanced manufacturing capabilities, and rising adoption of viral vector-based therapies in hospitals and research centers
- Asia-Pacific is expected to be the fastest-growing region in the gammaretroviral vector market during the forecast period, with a CAGR of 9.3% from 2025 to 2032. Growth in the region is fueled by expanding healthcare infrastructure, increasing R&D investments, rising government initiatives to promote gene therapy, and growing awareness about advanced treatment options for genetic disorders in countries such as China, Japan, and India
- The Gene Therapy segment held the largest revenue share of 53.2% in 2024, fueled by the expanding use of viral vectors in treating genetic disorders, inherited diseases, and chronic conditions. Viral vectors serve as essential delivery tools for therapeutic genes, enabling precise correction of defective genes
Report Scope and Gammaretroviral Vector Market Segmentation
|
Attributes |
Gammaretroviral Vector Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Gammaretroviral Vector Market Trends
Emerging Trends in the Gammaretroviral Vector Market
- A significant and accelerating trend in the global gammaretroviral vector market is the growing focus on gene therapy applications, particularly for treating inherited immunodeficiencies, hematologic disorders, and certain cancers. Advanced research and clinical trials are driving the adoption of gammaretroviral vectors as reliable delivery systems for therapeutic genes
- Pharmaceutical and biotechnology companies are increasingly investing in next-generation vector development, aiming to enhance transduction efficiency, safety profiles, and long-term expression of therapeutic genes
- The market is witnessing innovations in vector engineering, including the development of self-inactivating vectors, tissue-specific promoters, and targeted delivery approaches, which improve therapeutic outcomes and minimize adverse effects
- Strategic collaborations between academic institutions, contract research organizations (CROs), and biotech firms are facilitating accelerated research, commercialization, and regulatory approval of gammaretroviral vector-based therapies
- Rising patient awareness and regulatory support for gene therapy solutions are contributing to market growth, with healthcare providers increasingly exploring personalized treatment strategies enabled by these vectors
- The demand for scalable manufacturing processes and improved quality control measures is fueling investments in vector production facilities, automation technologies, and standardized protocols, supporting broader clinical adoption and commercial viability of gammaretroviral vectors
Gammaretroviral Vector Market Dynamics
Driver
Growing Need Due to Expanding Gene Therapy and Vaccinology Applications
- The increasing prevalence of genetic disorders, infectious diseases, and the rising demand for targeted gene therapies is a significant driver for the heightened adoption of Gammaretroviral Vectors
- For instance, in 2023, several leading biopharmaceutical companies announced initiatives to enhance vector efficiency and safety, including collaboration programs for next-generation vector development. Such strategies by key companies are expected to drive the Gammaretroviral Vector industry growth in the forecast period
- As researchers and pharmaceutical companies focus on developing more effective gene therapy and vaccine delivery systems, Gammaretroviral Vectors provide a reliable platform for stable gene integration, offering significant therapeutic potential
- Furthermore, the growing emphasis on personalized medicine and precision therapeutics is encouraging the use of Gammaretroviral Vectors for patient-specific gene modifications, expanding their applicability across multiple disease areas
- The scalability of vector production, improvements in transduction efficiency, and enhanced safety profiles are key factors propelling the adoption of Gammaretroviral Vectors in both clinical and research settings. Increased awareness among healthcare providers and researchers further contributes to market growth
Restraint/Challenge
Concerns Regarding Safety and High Manufacturing Costs
- Safety concerns related to insertional mutagenesis and off-target effects remain a significant challenge for the broader adoption of gammaretroviral vectors. The presence of these risks necessitates stringent regulatory oversight, as gene therapy trials must undergo rigorous validation processes. This regulatory rigor can slow product adoption, requiring companies to demonstrate robust clinical safety data before widespread acceptance
- Manufacturing complexities present another key barrier to market growth. The production of high-quality viral vectors demands specialized facilities, advanced equipment, and strict quality control measures. These requirements result in elevated production costs, making Gammaretroviral Vectors relatively expensive compared to alternative delivery systems, which can limit adoption by cost-sensitive institutions
- Addressing these challenges through advanced vector engineering, optimized manufacturing processes, and comprehensive clinical safety evaluations is critical for obtaining regulatory approvals and gaining the confidence of end-users. Companies are increasingly investing in scalable production platforms and automation technologies to reduce costs while ensuring consistent vector quality and efficacy
- Limited accessibility in emerging markets further restricts adoption, as the high costs and infrastructure demands of Gammaretroviral Vector production can pose challenges for academic and smaller research institutes. This limitation underscores the need for strategies to enhance distribution and availability in these regions
- Overcoming these barriers through innovation in vector design, cost-efficient manufacturing, and comprehensive training programs for researchers and clinicians will be essential for sustained market growth. Such initiatives can help expand the reach of Gammaretroviral Vectors, improve safety standards, and build long-term confidence among users
Gammaretroviral Vector Market Scope
The Gammaretroviral Vector market is segmented on the basis of type, application, and end-user.
- By Type
On the basis of type, the gammaretroviral vector market is segmented into replication-competent and replication-defective vectors. The Replication-Competent segment dominated the market with a revenue share of 46.1% in 2024, driven by their ability to efficiently deliver genetic material and induce strong therapeutic effects in target cells. These vectors are extensively used in research and clinical settings for immunotherapy, CAR-T cell production, and experimental gene therapy applications. Their high transduction efficiency, stable gene expression, and suitability for multiple therapeutic areas contribute to their market dominance. Continuous technological advancements and optimization of replication-competent vectors for enhanced safety and reduced cytotoxicity further fuel their adoption. Pharmaceutical and biotechnology companies are actively investing in the development and large-scale production of these vectors to support pipeline therapies. Their versatility across diverse clinical indications reinforces their leading position in the market.
The Replication-Defective segment is expected to witness the fastest CAGR of 18.9% from 2025 to 2032, driven by the growing focus on safety and controlled gene delivery in clinical applications. These vectors are preferred in situations where transient expression is required or where minimizing viral replication is critical. They are increasingly utilized in gene therapy programs targeting rare genetic disorders and cancer immunotherapies due to their reduced pathogenicity and lower risk profile. Regulatory approvals for replication-defective viral vectors in clinical trials are increasing, which encourages their adoption in both research and commercial therapeutic applications. Advances in vector engineering and scalable manufacturing technologies further enhance the appeal of replication-defective systems. The ability to customize these vectors for specific tissues and applications supports rapid market growth.
- By Application
On the basis of application, the gammaretroviral vector market is segmented into gene therapy, cancer therapy, vaccines, and others. The Gene Therapy segment held the largest revenue share of 53.2% in 2024, fueled by the expanding use of viral vectors in treating genetic disorders, inherited diseases, and chronic conditions. Viral vectors serve as essential delivery tools for therapeutic genes, enabling precise correction of defective genes. Increasing approvals of gene therapy products and the rising pipeline of experimental therapies support this segment’s dominance. Biopharmaceutical companies are investing heavily in vector-based gene therapy programs, and technological advancements have improved transduction efficiency and safety profiles. The segment’s versatility across multiple diseases ensures continued market leadership.
The Cancer Therapy segment is anticipated to witness the fastest CAGR of 17.5% from 2025 to 2032, driven by the rising prevalence of solid tumors and hematologic malignancies globally. Viral vectors are critical for developing CAR-T cells, oncolytic therapies, and immunomodulatory gene therapies in oncology. Increasing research funding, government support, and clinical trial activity targeting cancer therapies boost the adoption of gammaretroviral vectors in this application. Technological innovations in vector design and combination approaches with immunotherapies further enhance treatment efficacy. Collaboration between academic institutions and pharmaceutical companies accelerates research and commercial adoption. The growing demand for personalized and targeted cancer therapies reinforces the segment’s rapid growth trajectory.
- By End-User
On the basis of end-user, the gammaretroviral vector market is segmented into research institutes, biotechnology companies, pharmaceutical companies, and others. The Pharmaceutical Companies segment dominated with a revenue share of 58.4% in 2024, supported by their extensive capabilities in large-scale production, commercialization of viral vector-based therapies, and investments in research pipelines. These companies focus on developing gene therapies, cancer therapeutics, and vaccines, leveraging viral vectors as critical delivery platforms. Strong regulatory support, clinical trial approvals, and collaborations with research institutes enhance market dominance. Increasing demand for high-quality, scalable vector production and commercialization infrastructure further drives growth.
The Research Institutes segment is expected to witness the fastest CAGR of 18.2% from 2025 to 2032, fueled by the expansion of academic research, preclinical studies, and experimental gene therapy programs. Institutes are increasingly focused on improving vector safety, transduction efficiency, and delivery precision. Collaborations with biotechnology and pharmaceutical companies enhance funding opportunities and practical applications of viral vectors. The growing interest in rare disease therapeutics, experimental cancer therapies, and innovative gene therapy platforms contributes to accelerated adoption. Research institutes also play a pivotal role in developing new viral vector systems, optimizing them for clinical translation, and supporting preclinical validation, which ensures sustained market growth.
Gammaretroviral Vector Market Regional Analysis
- North America dominated the gammaretroviral vector market with the largest revenue share of 41.5% in 2024, driven by strong biotechnology infrastructure, high R&D investments, and leading pharmaceutical and gene therapy companies. The region benefits from increasing clinical trials, advanced manufacturing, and rising adoption of viral vector therapies in hospitals and research centers
- Collaboration between industry and academic institutions strengthens innovation and commercialization of gene therapies. Growth is supported by government funding, regulatory frameworks, and focus on precision medicine
- Expansion of biopharmaceutical facilities ensures a reliable supply of viral vectors for therapeutic applications. North America remains a hub for research, development, and adoption of viral vector technologies
U.S. Gammaretroviral Vector Market Insight
The U.S. gammaretroviral vector market captured the largest share within North America. Market growth is fueled by advanced clinical trials, hospital adoption of viral vector therapies, and strong R&D investments. Leading pharmaceutical and biopharmaceutical companies are developing gene therapies for cancer, genetic disorders, and infectious diseases. Government initiatives and regulatory support facilitate faster clinical development and commercialization. Research institutes and hospitals increasingly integrate viral vector technologies into treatment and research. The U.S. serves as a global leader in viral vector innovation, production, and adoption.
Europe Gammaretroviral Vector Market Insight
The Europe gammaretroviral vector market shows steady growth due to strong healthcare infrastructure, gene therapy clinical trials, and biotechnology investments. Germany, France, and the U.K. contribute via research initiatives and academic-industry collaborations. Hospitals and research institutes are increasing adoption for cancer and genetic disorder therapies. Regulatory support for gene therapy trials accelerates market expansion. Focus on personalized medicine and precision therapeutics drives adoption. The region’s biotechnology ecosystem supports sustainable growth.
U.K. Gammaretroviral Vector Market Insight
The U.K gammaretroviral vector market. is driven by gene therapy research, rising demand for precision medicine, and robust healthcare infrastructure. Hospitals and research institutes are using viral vectors for cancer and genetic disorder studies. Government initiatives and academic-industry collaborations support market expansion. Increasing adoption in clinical trials boosts the demand for viral vector technologies. The biotechnology sector ensures continued market growth.The U.K. remains a strong contributor to the European market.
Germany Gammaretroviral Vector Market Insight
The Germany gammaretroviral vector market is growing due to advanced biotechnology infrastructure and regulatory support for gene therapy. Hospitals and research institutes increasingly adopt viral vectors for cancer and genetic disorders. Biopharmaceutical companies leverage local manufacturing for vector production. Integration of gene therapies into treatment regimens drives demand. R&D investments and academic-industry collaborations support growth. Germany maintains a strong presence in the European market.
Asia-Pacific Gammaretroviral Vector Market Insight
The Asia-Pacific gammaretroviral vector market is the fastest-growing region with a CAGR of 9.3% from 2025 to 2032. Growth is fueled by expanding healthcare infrastructure, increasing R&D investments, and rising gene therapy awareness in China, Japan, and India. Hospitals, research institutes, and biopharmaceutical companies are adopting viral vectors for clinical and research applications. Government incentives and biotechnology hubs support market expansion. Domestic manufacturing enhances affordability and accessibility. The region is emerging as a key driver of global market growth.
Japan Gammaretroviral Vector Market Insight
The Japan gammaretroviral vector market grows due to biotechnology investments, advanced healthcare infrastructure, and government support for gene therapy. Hospitals and research institutes adopt viral vectors for cancer and genetic disorder therapies. Collaborations between domestic and international companies enhance vector development. Aging population and personalized medicine demand drive adoption. Manufacturing capabilities ensure consistent supply. Japan contributes significantly to Asia-Pacific market growth.
China Gammaretroviral Vector Market Insight
The China gammaretroviral vector market accounted for the largest Asia-Pacific revenue share in 2024, driven by biotechnology investments and gene therapy awareness. Hospitals, research institutes, and biopharmaceutical companies adopt viral vectors for clinical and research use. Government initiatives promote biotechnology and gene therapy innovation. Domestic vector manufacturing improves affordability and accessibility. Increasing clinical trials and research projects support adoption. China remains a key growth driver in the region.
Gammaretroviral Vector Market Share
The Gammaretroviral Vector industry is primarily led by well-established companies, including:
- Novasep (France)
- Merck KGaA (Germany)
- Charles River Laboratories (U.K.)
- uniQure N.V. (Netherlands)
- Waisman Biomanufacturing (U.S.)
- Creative-Biogene (U.S.)
- Aldevron (U.S.)
- Addgene (U.S.)
- Oxford Biomedica (U.K.)
- Thermo Fisher Scientific Inc (U.S.)
- Fujifilm Corporation (Japan)
- ABL Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Brammer Bio (U.S.)
- Creative Biogene (U.S.)
Latest Developments in Global Gammaretroviral Vector Market
- In August 2025, Novasep, a leading provider of viral vector manufacturing services, announced advancements in its Gammaretroviral Vector production capabilities. The company highlighted its commitment to enhancing the safety and efficiency of gene therapy products. Novasep's efforts focus on improving vector design and manufacturing processes to meet the growing demand for gene therapies. This development underscores the company's role in supporting the expansion of the Gammaretroviral Vector market
- In August 2025, Merck KGaA, Darmstadt, Germany, emphasized its ongoing research and development in the field of Gammaretroviral Vectors. The company is actively exploring innovations to enhance the safety profiles of these vectors, particularly in the context of gene therapy applications. Merck KGaA's initiatives aim to address challenges such as insertional mutagenesis and to improve the overall efficacy of gene delivery systems
- In August 2025, Charles River Laboratories reported progress in its Gammaretroviral Vector services, focusing on optimizing vector production for gene therapy applications. The company is working on refining manufacturing processes to ensure scalability and compliance with regulatory standards. Charles River Laboratories' efforts are directed towards supporting the clinical development of gene therapies that utilize Gammaretroviral Vectors
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.