Global Gliflozin Market
Market Size in USD Billion
CAGR :
% 
 USD
6.69 Billion
USD
12.20 Billion
2024
2032
USD
6.69 Billion
USD
12.20 Billion
2024
2032
| 2025 –2032 | |
| USD 6.69 Billion | |
| USD 12.20 Billion | |
|
|
|
|
Gliflozin Market Size
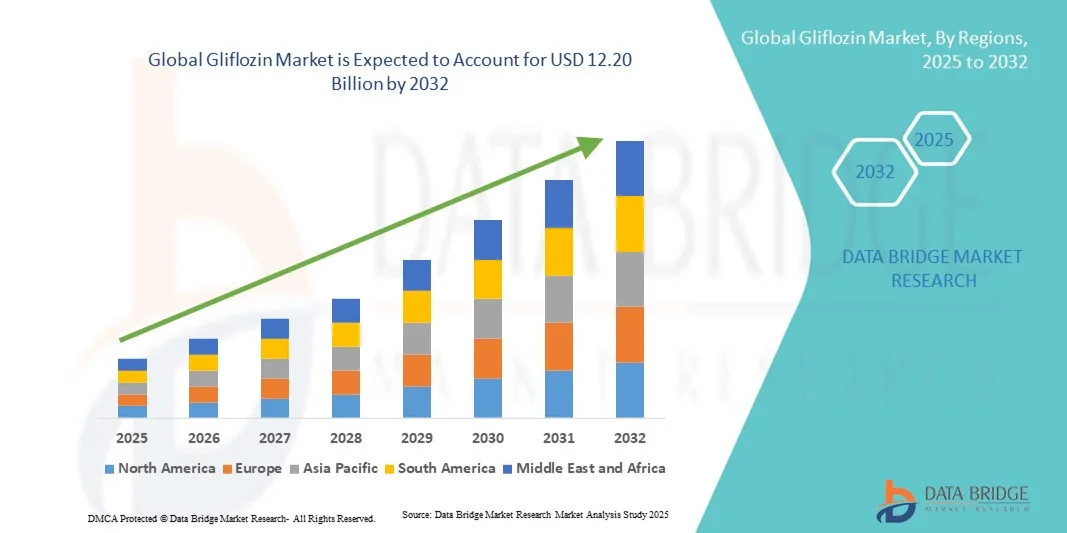
- The global gliflozin market size was valued at USD 6.69 billion in 2024 and is expected to reach USD 12.20 billion by 2032, at a CAGR of 7.80% during the forecast period
- The market growth is largely fueled by the rising prevalence of type 2 diabetes and the expanding use of SGLT2 inhibitors in managing cardiovascular and renal complications, driven by the growing emphasis on effective and multifunctional therapeutic options for chronic diseases
- Furthermore, increasing regulatory approvals, broader clinical adoption, and rising patient awareness regarding the metabolic and cardiovascular benefits of gliflozins are positioning these drugs as a preferred therapeutic class. These converging factors are accelerating the uptake of gliflozin-based treatments, thereby significantly boosting the industry’s growth
Gliflozin Market Analysis
- Gliflozins, a class of sodium-glucose co-transporter 2 (SGLT2) inhibitors, are increasingly vital in the management of type 2 diabetes and related cardiovascular and renal conditions, offering dual benefits of glycemic control and organ protection, making them a cornerstone of modern diabetes therapy
- The escalating demand for gliflozins is primarily fueled by the rising global prevalence of diabetes, growing clinical evidence supporting their cardioprotective and renoprotective effects, and increasing adoption of combination treatments that enhance patient adherence and long-term outcomes
- North America dominated the gliflozin market with the largest revenue share of 38.5% in 2024, driven by early regulatory approvals, strong pharmaceutical presence, and high awareness among healthcare professionals, with the U.S. leading usage of branded products such as Jardiance, Farxiga, and Invokana
- Asia-Pacific is expected to be the fastest growing region in the gliflozin market during the forecast period due to the rapid rise in diabetes incidence, improving access to advanced therapies, and growing healthcare investments in emerging economies such as China and India
- The Jardiance segment dominated the gliflozin market with a market share of 41.2% in 2024, attributed to its proven cardiovascular and renal benefits, favorable safety profile, and strong physician preference, despite the potential side effects such as urinary tract infections, yeast infections, and hypotension
Report Scope and Gliflozin Market Segmentation
|
Attributes |
Gliflozin Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Gliflozin Market Trends
Expansion Beyond Glycemic Control Through Cardiovascular and Renal Benefits
- A significant and accelerating trend in the global gliflozin market is the expanding use of SGLT2 inhibitors beyond blood glucose regulation, particularly in cardiovascular and renal disease management. This diversification of therapeutic applications is reshaping the drug class’s clinical relevance and driving stronger adoption across multiple specialties
- For instance, empagliflozin and dapagliflozin have demonstrated significant reductions in cardiovascular mortality and hospitalization for heart failure in both diabetic and non-diabetic patients, resulting in broader clinical usage and revised treatment guidelines
- The growing number of clinical trials investigating gliflozins for kidney protection and heart failure with preserved ejection fraction (HFpEF) reflects a major shift toward multifunctional disease management. Furthermore, these drugs are being studied for potential anti-inflammatory and metabolic benefits that extend their therapeutic horizon
- The expanding research and integration of gliflozins into multidisciplinary treatment frameworks are allowing healthcare providers to manage complex comorbidities through a single pharmacological approach, improving patient outcomes and adherence
- This trend toward broader indication approval and real-world clinical validation is fundamentally reshaping the competitive dynamics of the pharmaceutical market. Consequently, leading companies such as AstraZeneca and Boehringer Ingelheim are investing heavily in large-scale studies and label expansions to strengthen their market presence
- The demand for gliflozin drugs that offer comprehensive cardiovascular and renal protection is growing rapidly across developed and emerging markets, as physicians increasingly prioritize holistic, evidence-based treatment strategies
Gliflozin Market Dynamics
Driver
Rising Global Diabetes Burden and Expanding Therapeutic Indications
- The increasing global prevalence of type 2 diabetes, coupled with growing recognition of SGLT2 inhibitors as effective agents for cardiovascular and renal protection, is a significant driver for the heightened demand for gliflozin drugs
- For instance, in March 2024, AstraZeneca reported expanding clinical adoption of Farxiga (dapagliflozin) for chronic kidney disease and heart failure management, supported by new international treatment guidelines endorsing its broader use. Such advancements are expected to drive market growth during the forecast period
- As the global diabetic population continues to rise, the need for medications that provide multifaceted benefits beyond glucose control is creating substantial opportunities for gliflozin therapies
- Furthermore, the growing physician preference for early adoption of SGLT2 inhibitors and their incorporation into standard care protocols are reinforcing their position as essential components in diabetes and comorbidity management
- The proven efficacy of gliflozins in reducing hospitalization, slowing disease progression, and improving quality of life are key factors propelling their adoption across both primary and specialty care settings. The ongoing expansion of patient access programs and healthcare awareness initiatives further contribute to market growth
- Collaborations between global health organizations and drug manufacturers to enhance accessibility and affordability of gliflozins in low- and middle-income countries are also strengthening long-term market growth potential
Restraint/Challenge
Adverse Effects and Stringent Regulatory Compliance Requirements
- Concerns surrounding the side effects of gliflozin drugs, including urinary tract infections, genital yeast infections, and diabetic ketoacidosis, pose a significant challenge to broader market acceptance. These safety concerns require continuous post-marketing surveillance and patient education
- For instance, several regulatory bodies have issued advisories urging close monitoring of patients on gliflozin therapy due to rare but serious occurrences of euglycemic ketoacidosis, which can affect long-term compliance and physician prescribing behavior
- Addressing these safety and compliance challenges through transparent reporting, real-world evidence generation, and robust pharmacovigilance programs is crucial for maintaining physician and patient confidence. Companies such as Janssen and Merck are emphasizing risk mitigation strategies and ongoing research to support safety assurance
- In addition, varying regulatory approval timelines and the need for extensive cardiovascular outcome data can delay market entry for new formulations, particularly in emerging economies with stricter evidence requirements. While awareness and clinical familiarity are improving, regulatory and safety hurdles still pose barriers to rapid global expansion
- Overcoming these challenges through continuous innovation, patient-centric education, and stronger collaboration between pharmaceutical firms and regulatory agencies will be vital for the sustained growth of the gliflozin market
- The limited availability of long-term real-world safety data in diverse patient populations, including elderly and comorbid individuals, continues to restrict broader adoption until further evidence validates long-term efficacy and tolerability
Gliflozin Market Scope
The market is segmented on the basis of type and side effects.
- By Type
On the basis of type, the global gliflozin market is segmented into Invokana, Farxiga, Jardiance, and Steglatro. The Jardiance segment dominated the market with the largest market revenue share of 41.2% in 2024, attributed to its strong clinical evidence supporting cardiovascular and renal benefits beyond glycemic control. Its success is largely driven by widespread physician preference and inclusion in international treatment guidelines for both diabetic and non-diabetic patients. Jardiance’s extensive approval coverage across major markets, including the U.S. and Europe, enhances its commercial performance. Furthermore, its strong collaboration between Boehringer Ingelheim and Eli Lilly has resulted in robust brand visibility and expanding patient access programs. The growing emphasis on evidence-based diabetes care and positive real-world data further consolidate Jardiance’s leading position in the gliflozin market.
The Farxiga segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by its expanding therapeutic use in chronic kidney disease (CKD) and heart failure management, regardless of diabetic status. Farxiga (dapagliflozin) has been at the forefront of label expansion initiatives, gaining regulatory approvals from the U.S. FDA and EMA for multiple indications. The increasing physician trust in its safety and efficacy profile and growing penetration in emerging markets are propelling its adoption. AstraZeneca’s aggressive global marketing strategy, combined with strong clinical data from landmark trials such as DAPA-HF and DAPA-CKD, continues to drive growth. Rising awareness of its dual benefits in metabolic and cardiovascular conditions positions Farxiga as the fastest-growing gliflozin product globally.
- By Side Effects
On the basis of side effects, the gliflozin market is segmented into urinary tract infections, yeast infections, diabetic ketoacidosis, hypoglycemia, and hypotension. The urinary tract infections (UTIs) segment dominated the market in 2024, as it remains the most frequently reported side effect associated with gliflozin therapy due to the increased glucose concentration in urine that fosters bacterial growth. Despite being mild to moderate in most cases, the high occurrence rate of UTIs necessitates patient monitoring and education, especially among women and older adults. Pharmaceutical companies have responded by emphasizing preventive guidance and optimized dosage strategies to mitigate these risks. The dominance of this segment underscores the need for improved formulation strategies and ongoing safety surveillance. Continued real-world evidence collection is expected to enhance understanding of the correlation between gliflozin dosage, duration, and infection risk.
The hypotension segment is projected to witness the fastest growth during the forecast period, driven by the increased clinical use of gliflozins among patients with heart failure and advanced chronic kidney disease, where blood pressure management is critical. As SGLT2 inhibitors promote osmotic diuresis and natriuresis, they can lead to volume depletion and transient drops in blood pressure, especially in elderly or poly-medicated patients. The growing use of gliflozins in high-risk cardiovascular patients has resulted in heightened reporting of hypotensive events, prompting the need for tailored dosing and patient monitoring protocols. However, awareness and improved clinical management are expected to reduce severe cases. This segment’s rise highlights the ongoing challenge of balancing therapeutic efficacy with patient safety as gliflozin adoption continues to expand globally.
Gliflozin Market Regional Analysis
- North America dominated the gliflozin market with the largest revenue share of 38.5% in 2024, driven by early regulatory approvals, strong pharmaceutical presence, and high awareness among healthcare professionals, with the U.S. leading usage of branded products such as Jardiance, Farxiga, and Invokana
- Consumers and healthcare providers in the region highly value the proven cardiovascular and renal benefits, favorable safety profile, and broad availability of gliflozin-based treatments such as Jardiance, Farxiga, and Invokana
- This widespread adoption is further supported by favorable reimbursement frameworks, extensive clinical research activities, and increasing physician preference for evidence-based, multifaceted diabetes management, establishing gliflozins as a preferred therapeutic option across North America
U.S. Gliflozin Market Insight
The U.S. gliflozin market captured the largest revenue share of 79% in 2024 within North America, driven by the high prevalence of type 2 diabetes and early adoption of advanced SGLT2 inhibitors such as Jardiance, Farxiga, and Invokana. Strong physician awareness, favorable reimbursement policies, and active clinical research have accelerated their uptake across diabetic and cardiovascular care segments. Consumers and healthcare providers increasingly favor gliflozins for their dual benefits in glycemic and organ protection. Moreover, growing regulatory support and widespread marketing initiatives by key players such as AstraZeneca and Boehringer Ingelheim continue to propel market expansion in the U.S.
Europe Gliflozin Market Insight
The Europe gliflozin market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising cases of diabetes and a growing focus on cardiovascular and renal health. The region’s stringent medical guidelines emphasizing evidence-based therapies have strengthened gliflozin adoption. Increasing healthcare expenditure and government support for innovative diabetes care solutions are further supporting market growth. European consumers and physicians are drawn to gliflozin’s safety and long-term benefits, leading to higher uptake across hospitals, clinics, and pharmacies.
U.K. Gliflozin Market Insight
The U.K. gliflozin market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by increasing diabetes prevalence and rising clinical preference for SGLT2 inhibitors in both primary and specialist care. The National Institute for Health and Care Excellence (NICE) has issued favorable recommendations supporting gliflozin use, driving broader adoption. Furthermore, heightened awareness regarding cardiovascular risk reduction and strong availability through the National Health Service (NHS) are boosting market penetration. The U.K.’s advanced healthcare infrastructure and patient access programs are expected to sustain strong growth momentum.
Germany Gliflozin Market Insight
The Germany gliflozin market is expected to expand at a considerable CAGR during the forecast period, supported by high healthcare standards, proactive disease management, and early adoption of innovative therapeutics. German physicians emphasize personalized treatment strategies, making gliflozins a preferred option for diabetes with comorbid cardiovascular risks. Favorable reimbursement coverage, combined with strong R&D presence of key pharmaceutical companies, continues to enhance market visibility. Moreover, the growing number of clinical trials and patient education initiatives on SGLT2 inhibitor benefits are fueling further adoption in Germany.
Asia-Pacific Gliflozin Market Insight
The Asia-Pacific gliflozin market is poised to grow at the fastest CAGR of 22% during 2025–2032, driven by surging diabetes prevalence, improving healthcare access, and expanding awareness of gliflozin’s cardiometabolic benefits. Countries such as China, Japan, and India are witnessing significant growth due to the rising middle-class population and government-led health initiatives promoting advanced diabetes treatments. The affordability of generic variants and expanding distribution networks are increasing accessibility. Moreover, growing collaborations between global and local pharmaceutical firms are accelerating gliflozin adoption across the region.
Japan Gliflozin Market Insight
The Japan gliflozin market is gaining momentum due to the country’s aging population, rising incidence of type 2 diabetes, and high healthcare awareness. The integration of gliflozins into national treatment guidelines has encouraged widespread clinical use, particularly in combination therapies. For instance, products such as Luseogliflozin and Ipragliflozin have achieved strong local market penetration. In addition, Japan’s emphasis on precision medicine and continuous patient monitoring aligns with the preventive benefits offered by gliflozin drugs. This growing focus on holistic disease management continues to propel demand across both hospital and outpatient settings.
India Gliflozin Market Insight
The India gliflozin market accounted for the largest market revenue share in Asia Pacific in 2024, supported by the country’s expanding diabetic population, rising healthcare investments, and strong presence of generic manufacturers. Increasing physician confidence in gliflozin-based therapy, along with the availability of affordable branded and generic options, is enhancing accessibility across urban and semi-urban regions. The government’s initiatives toward diabetes awareness and preventive care are further promoting adoption. Moreover, partnerships between domestic and multinational companies are strengthening supply chains, making India one of the most promising growth markets for gliflozin drugs.
Gliflozin Market Share
The Gliflozin industry is primarily led by well-established companies, including:
- AstraZeneca (U.K.)
- Merck & Co., Inc., (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Sanofi (France)
- Eli Lilly and Company (U.S.)
- Lexicon Pharmaceuticals (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Cipla (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Lupin (India)
- Torrent Pharmaceuticals Ltd. (India)
- GLENMARK PHARMACEUTICALS LTD. (India)
- Boehringer Ingelheim International GmbH (Germany).
- Pfizer Inc. (U.S.).
- Viatris Inc. (U.S.).
- Aurobindo Pharma Limited (India).
- Zydus Group (India).
- Hetero (India).
- Alembic Pharmaceuticals Limited (India)
- Macleods Pharmaceuticals Ltd. (India)
What are the Recent Developments in Global Gliflozin Market?
- In April 2025, following patent expiry of Jardiance’s empagliflozin in India (March 11 2025), there was a rapid surge of generic and branded-generic versions: 71 new brands from 17 companies entered the market within a month, and prices dropped by 80-90%. This development illustrates the impact of patent expiry on accessibility and pricing, especially in emerging markets
- In June 2024, the FDA approved Farxiga (dapagliflozin) for use in paediatric patients (10 years and older) with type 2 diabetes mellitus (T2D). This represented one of the first approvals of an SGLT2 inhibitor in children for T2D, a segment with growing prevalence and unmet needs
- In May 2023, Farxiga (dapagliflozin) received FDA sanction to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visits across the full spectrum of left ventricular ejection fraction (any LVEF) in adults with heart failure. The approval reflected results from the DELIVER trial and marked the first SGLT2 inhibitor with such broad heart-failure indication
- In February 2022, the FDA expanded approval of Jardiance (empagliflozin) for adults with heart failure regardless of left ventricular ejection fraction (LVEF), thereby extending its use beyond HFrEF to heart failure with preserved ejection fraction (HFpEF). This was supported by the EMPEROR-Preserved trial which demonstrated a 21% relative risk reduction for the composite of cardiovascular death or hospitalization for heart failure
- In August 2021, Jardiance (empagliflozin) received U.S. Food and Drug Administration (FDA) approval for use in adults with heart failure with reduced ejection fraction (HFrEF) — the first SGLT2 inhibitor approved for that indication. The decision was based on the phase III EMPEROR-Reduced trial which showed a ~25% reduction in risk of cardiovascular death or hospitalization for heart failure versus placebo
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.