Global Gm1 Gangliosidosis Market
Market Size in USD Billion
CAGR :
% 
 USD
1.11 Billion
USD
7.27 Billion
2024
2032
USD
1.11 Billion
USD
7.27 Billion
2024
2032
| 2025 –2032 | |
| USD 1.11 Billion | |
| USD 7.27 Billion | |
|
|
|
|
GM1 Gangliosidosis Market Size
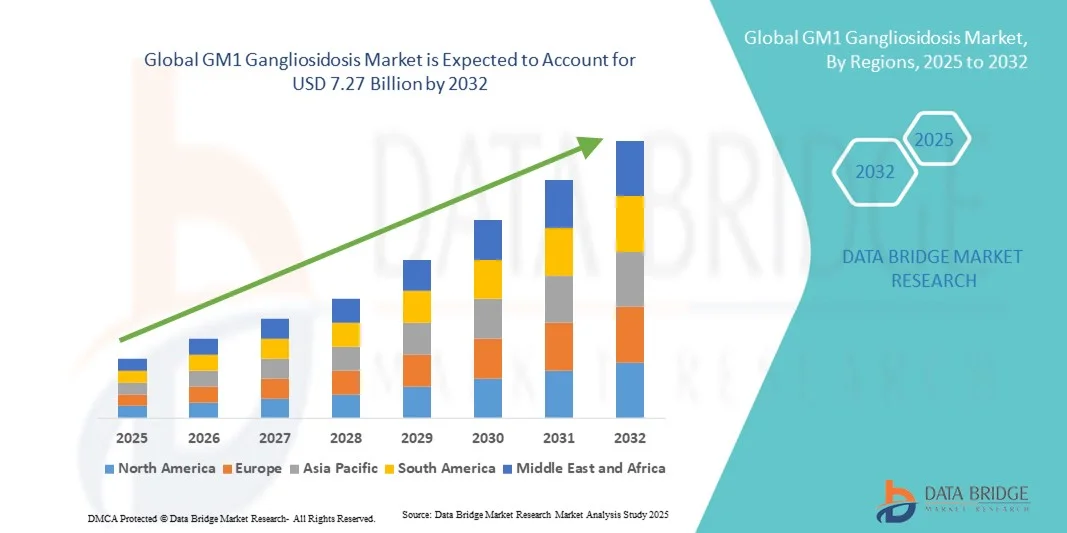
- The global GM1 gangliosidosis market size was valued at USD 1.11 billion in 2024 and is expected to reach USD 7.27 billion by 2032, at a CAGR of 26.50% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within connected home devices and smart home technology, leading to increased digitalization in both residential and commercial settings
- Furthermore, rising consumer demand for secure, user-friendly, and integrated solutions for their homes and businesses is establishing smart locks as the modern access control system of choice. These converging factors are accelerating the uptake of GM1 Gangliosidosis solutions, thereby significantly boosting the industry's growth
GM1 Gangliosidosis Market Analysis
- GM1 gangliosidosis, a rare genetic lysosomal storage disorder, is increasingly drawing attention due to rising awareness, advancements in gene therapy, and growing focus on orphan drug development for effective disease management
- The escalating demand for innovative treatments is primarily fueled by increased R&D investments, growing patient advocacy, and expanding access to specialized healthcare facilities for rare genetic disorders
- North America dominated the GM1 gangliosidosis market with the largest revenue share of 42.3% in 2024, driven by advanced healthcare infrastructure, high healthcare spending, and the presence of leading pharmaceutical companies focusing on rare disease therapies, particularly in the U.S., which continues to lead in development and adoption of novel treatment protocols
- Asia-Pacific is expected to be the fastest-growing region in the GM1 gangliosidosis market during the forecast period, with a projected CAGR of 23.7%, fueled by increasing healthcare awareness, improving access to rare disease treatments, and rising investments in genetic disorder research across countries like India, China, and Japan
- The Classic Infantile segment dominated the largest market revenue share of 45.6% in 2024, owing to its early onset and severe progression, which necessitates immediate medical attention and advanced therapeutic interventions
Report Scope and GM1 Gangliosidosis Market Segmentation
|
Attributes |
GM1 Gangliosidosis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
GM1 Gangliosidosis Market Trends
Rising Focus on Early Diagnosis and Therapeutic Advancements
- A significant and accelerating trend in the global GM1 gangliosidosis market is the increasing emphasis on early diagnosis and the development of innovative therapies. Advances in genetic testing, enzyme replacement therapies, and substrate reduction treatments are enabling earlier detection and more effective management of GM1 gangliosidosis, thereby significantly improving patient outcomes
- For instance, in July 2023, Lysogene, a leading biotechnology company, announced the initiation of a Phase II/III clinical trial for a novel gene therapy targeting GM1 gangliosidosis, aiming to address neurological symptoms and slow disease progression. Such developments are expected to transform disease management and expand therapeutic options for patients.
- Integration of advanced diagnostics with personalized medicine approaches is becoming increasingly prevalent, allowing physicians to tailor treatment regimens based on individual genetic and clinical profiles. This shift is enhancing patient monitoring, optimizing treatment efficacy, and reducing adverse effects
- Research collaborations between academic institutions, biotechnology firms, and pharmaceutical companies are accelerating the development of next-generation therapies. These partnerships focus on leveraging cutting-edge molecular biology techniques, CRISPR-based interventions, and targeted small-molecule therapies to improve disease outcomes
- The growing awareness of GM1 gangliosidosis among clinicians, caregivers, and patient advocacy groups is further driving demand for innovative therapeutic solutions. Public and private investments in rare disease research, along with expanded patient registries, are providing critical data to guide therapy development
- Regulatory agencies are increasingly providing accelerated approval pathways and orphan drug designations, which facilitate faster market access for promising GM1 Gangliosidosis therapies, thereby encouraging continued innovation in the field
GM1 Gangliosidosis Market Dynamics
Driver
Rising Investment in Rare Disease Research and Development
- Increasing funding from government bodies, private investors, and global health organizations is a key driver for the GM1 Gangliosidosis market, enabling accelerated research into disease mechanisms and potential therapies
- For instance, in March 2022, the National Institutes of Health (NIH) awarded a grant to support research on novel gene therapy approaches for lysosomal storage disorders, including GM1 Gangliosidosis, fostering innovation in treatment development
- The expansion of patient registries and natural history studies provides critical data that informs clinical trial design, enhances understanding of disease progression, and accelerates regulatory approvals
- Advances in biotechnology and molecular medicine are enabling the development of precision therapies tailored to specific genetic mutations associated with GM1 Gangliosidosis, improving treatment efficacy
- Collaboration between pharmaceutical companies, biotechnology firms, and academic researchers is enhancing knowledge sharing and speeding up the translation of laboratory discoveries into clinical applications
- Increased awareness among healthcare professionals and caregivers about emerging therapies is contributing to higher adoption rates and encouraging earlier intervention strategies
Restraint/Challenge
High Treatment Costs and Limited Access to Specialized Care
- The high cost of advanced GM1 gangliosidosis therapies, particularly gene therapies and enzyme replacement treatments, presents a significant challenge to market growth, limiting accessibility for many patients worldwide
- For instance, reports indicate that single-dose gene therapies for rare lysosomal storage disorders can exceed millions of dollars, posing affordability barriers even in developed healthcare systems
- Access to specialized diagnostic facilities and treatment centers remains limited in emerging economies, resulting in delayed diagnosis and treatment initiation
- Regulatory and reimbursement complexities across different regions can impede timely approval and adoption of novel therapies, restricting patient access
- The rarity of GM1 Gangliosidosis creates challenges in patient recruitment for clinical trials, slowing down therapy development and market introduction
- Addressing these challenges requires strategies such as expanded insurance coverage, government subsidies, global patient assistance programs, and investment in regional treatment centers to ensure equitable access to care
GM1 Gangliosidosis Market Scope
The market is segmented on the basis of type, diagnosis, treatment, end-user, and distribution channel.
- By Type
On the basis of type, the GM1 Gangliosidosis market is segmented into Classic Infantile, Juvenile, and Adult. The Classic Infantile segment dominated the largest market revenue share of 45.6% in 2024, owing to its early onset and severe progression, which necessitates immediate medical attention and advanced therapeutic interventions. Early diagnosis in infants has prompted rapid adoption of enzyme replacement therapies, gene therapies, and supportive treatments, contributing significantly to market revenue. Families and healthcare providers prioritize intensive monitoring and care for Classic Infantile GM1, increasing hospital visits and utilization of specialized treatment protocols. The high clinical focus on this type, coupled with established treatment guidelines and government-backed newborn screening programs in several regions, reinforces its dominance. The segment also benefits from ongoing research initiatives aimed at developing novel therapies and improving patient outcomes, attracting investment and funding. In addition, patient registries and increased awareness among pediatricians and neurologists drive early detection, further boosting market share. The urgency of treatment, high mortality risk, and concentration of research activities collectively strengthen the revenue dominance of the Classic Infantile segment.
The Juvenile segment is anticipated to witness the fastest growth rate of 19.8% CAGR from 2025 to 2032, driven by advancements in gene therapy and the expansion of diagnostic facilities enabling early detection. Increasing awareness among parents and clinicians about Juvenile GM1 and emerging therapies designed to slow disease progression are fueling market adoption. Improved access to pediatric neurology centers and enhanced healthcare infrastructure in emerging regions support this growth. Furthermore, collaborations between biotech firms and research institutions for clinical trials targeting Juvenile patients enhance market momentum. The relatively slower disease progression compared to the infantile form allows for multiple therapeutic interventions over time, which increases treatment utilization. Enhanced funding and patient support programs also accelerate adoption, while technological advancements in enzyme replacement therapy, combined with molecular genetic testing, further strengthen market penetration for the Juvenile segment.
- By Diagnosis
On the basis of diagnosis, the GM1 Gangliosidosis market is segmented into Enzyme Analysis, Molecular Genetic Testing, and Beta-Galactosidase Activity. The Enzyme Analysis segment held the largest market revenue share of 42.5% in 2024, driven by its reliability, established protocols, and widespread availability in hospitals and specialized diagnostic laboratories. Enzyme analysis allows early detection and accurate classification of GM1 types, enabling clinicians to select appropriate treatment plans. The technique is integral to newborn screening programs in developed countries, increasing sample throughput and hospital utilization. Enzyme-based diagnostics are often complemented by molecular genetic testing to confirm mutations, reinforcing clinical adoption. Established reimbursement frameworks and inclusion in pediatric metabolic disorder panels further support revenue dominance. Training and familiarity among clinical lab personnel, as well as rapid turnaround times for results, also contribute to the segment's leading market position. Standardization of enzyme activity assays and increasing investments in clinical laboratory infrastructure help maintain the segment’s dominance.
Molecular Genetic Testing is expected to witness the fastest CAGR of 20.4% from 2025 to 2032, propelled by technological advancements in sequencing, rising awareness of genetic counseling, and the increasing demand for precise mutation identification. Its non-invasive nature, accuracy in mutation detection, and ability to support personalized treatment strategies are driving adoption. Expansion of next-generation sequencing services and reduced costs of genetic tests make this segment highly attractive. The growing emphasis on early diagnosis and family planning among at-risk populations also fuels growth. Biotech companies are actively investing in genetic testing platforms, further accelerating market expansion. Regulatory support and inclusion of genetic tests in standard diagnostic protocols enhance adoption, while emerging research linking genotype to therapy response strengthens the growth potential for this segment.
- By Treatment
On the basis of treatment, the GM1 Gangliosidosis market is segmented into Anticonvulsants, Bone Marrow Transplantation, Cord-Blood Hematopoietic Stem-Cell Transplantation, Enzyme Replacement, and Gene Therapy. Enzyme Replacement Therapy (ERT) dominated the largest market revenue share of 44.8% in 2024, due to its ability to target the underlying enzyme deficiency and provide symptom management in pediatric and juvenile patients. ERT is widely adopted in hospitals and specialized centers, with increasing clinical trial activity supporting its use. Coverage by insurance and government programs enhances accessibility. Continuous innovations in delivery methods and dosing protocols improve patient compliance, while collaborations between biotech firms and hospitals expand availability. Early intervention protocols and growing awareness among clinicians and families also contribute to the segment’s dominance. Ongoing research aimed at improving blood-brain barrier penetration and enhancing efficacy further reinforces ERT’s leadership position.
Gene Therapy is expected to witness the fastest CAGR of 22.1% from 2025 to 2032, fueled by breakthroughs in viral vector design, CRISPR-based interventions, and increasing clinical trial initiatives targeting GM1 mutations. The promise of potentially curative outcomes drives high adoption in advanced research hospitals and specialized centers. Substantial investments by biotech companies and venture capital funding support rapid development. Regulatory agencies are increasingly granting orphan drug status and accelerated approvals, enhancing market entry prospects. Patient advocacy and rising awareness about genetic interventions also accelerate demand. The scalability of manufacturing processes and technological refinements in gene delivery strengthen market growth prospects.
- By End-User
On the basis of end-user, the GM1 Gangliosidosis market is segmented into Research Institutes, Hospitals, and Others. Hospitals accounted for the largest market revenue share of 46.2% in 2024, driven by their role in delivering diagnosis, treatment, and long-term care for GM1 patients. Hospitals provide infrastructure for enzyme analysis, stem-cell transplantation, and administration of advanced therapies, which increases utilization. Integration with academic research and clinical trials further boosts market revenue. Pediatric and neurology centers form the core of hospital adoption, with insurance coverage and government healthcare programs supporting treatment access. The presence of multidisciplinary teams enhances patient outcomes, reinforcing hospital dominance. Centralized facilities for rare disease management and ongoing collaboration with pharmaceutical and biotech firms maintain the segment’s market leadership.
Research Institutes are anticipated to witness the fastest CAGR of 19.9% from 2025 to 2032, driven by increasing funding for rare disease research, collaborations with biotech firms, and development of novel therapeutic approaches. Institutes are adopting advanced technologies such as gene editing, stem-cell research, and high-throughput screening to support drug discovery. Public and private research grants, along with partnerships with hospitals, accelerate clinical trials. Publication of findings in peer-reviewed journals and participation in international consortia enhance research impact. Expanding global research networks and the growing emphasis on translational medicine further strengthen growth prospects for this segment.
- By Distribution Channel
On the basis of distribution channel, the GM1 Gangliosidosis market is segmented into Hospitals and Others. Hospitals accounted for the largest market revenue share of 48.3% in 2024, as they serve as the primary channel for diagnostic tests, enzyme therapy administration, stem-cell procedures, and gene therapy applications. Hospitals provide integrated care with access to specialized physicians, lab facilities, and treatment infrastructure, ensuring consistent demand. Insurance coverage, reimbursement policies, and public health programs enhance hospital sales and utilization. Hospital dominance is reinforced by centralized procurement and patient management systems. The presence of dedicated rare disease centers increases adoption and market penetration. Hospitals are also involved in clinical trials, further boosting revenue.
The Others segment is expected to witness the fastest CAGR of 18.7% from 2025 to 2032, driven by emerging specialized diagnostic laboratories, biotech service providers, and home-care solutions supporting GM1 patients. Technological advancements, growing awareness, and partnerships with hospitals enable this segment to expand. The segment benefits from increasing accessibility in remote and underserved regions, while private clinics and telehealth initiatives contribute to rapid adoption. Investments in mobile diagnostic platforms and personalized medicine further accelerate growth. In addition, government incentives and grants for rare disease management are encouraging the establishment of new service providers in this segment. The increasing collaboration between global pharmaceutical companies and local distributors is also expected to enhance reach and improve patient access to advanced therapies.
GM1 Gangliosidosis Market Regional Analysis
- North America dominated the GM1 gangliosidosis market with the largest revenue share of 42.3% in 2024, driven by advanced healthcare infrastructure, high healthcare spending, and the presence of leading pharmaceutical companies focusing on rare disease therapies, particularly in the U.S., which continues to lead in development and adoption of novel treatment protocols
- The region benefits from well-established clinical research networks, advanced diagnostic facilities, and robust patient support programs, which collectively enhance the management of GM1 Gangliosidosis. The U.S., in particular, sees significant investment in gene therapy research, enzyme replacement therapies, and personalized medicine approaches, strengthening its market position
- High levels of healthcare expenditure, widespread access to specialized care centers, and strong collaborations between biotech firms and academic institutions further underpin North America’s dominance in the GM1 Gangliosidosis market. Patient advocacy groups and government initiatives are also increasing awareness and facilitating access to innovative therapies, supporting continuous market growth
U.S. GM1 Gangliosidosis Market Insight
The U.S. GM1 gangliosidosis market captured the largest revenue share within North America in 2024, driven by the country’s advanced healthcare system, early adoption of innovative therapeutic protocols, and strong presence of biotechnology and pharmaceutical companies specializing in rare genetic disorders. The U.S. is a key hub for clinical trials, research collaborations, and regulatory approvals for novel treatments, which accelerates the availability of effective therapies. Patient support initiatives, expanded insurance coverage, and specialized treatment centers further enhance access to care, reinforcing the U.S.’s leading position in the market.
Europe GM1 Gangliosidosis Market Insight
The Europe GM1 gangliosidosis market is projected to expand at a substantial CAGR throughout the forecast period, primarily supported by established healthcare systems, growing investment in rare disease research, and increasing availability of advanced treatment options. Countries such as Germany, France, and Italy are witnessing increased clinical trial activity and expanded access to therapies, which is improving diagnosis and treatment outcomes. The region’s regulatory frameworks and orphan drug incentives encourage pharmaceutical companies to develop and launch innovative therapies, driving market growth.
U.K. GM1 Gangliosidosis Market Insight
The U.K. GM1 gangliosidosis market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by rising awareness of rare genetic disorders, government support programs, and initiatives for early diagnosis and patient care. The NHS and specialized rare disease centers are facilitating access to advanced therapies, while patient advocacy groups are promoting early detection and intervention. Investments in clinical research and collaborative projects with pharmaceutical companies further contribute to market expansion.
Germany GM1 Gangliosidosis Market Insight
The Germany GM1 gangliosidosis market is expected to expand at a considerable CAGR during the forecast period, supported by high healthcare standards, increasing investment in biotechnology research, and adoption of innovative therapies. Germany’s well-developed healthcare infrastructure and strong focus on rare disease management encourage early diagnosis, clinical trials, and integration of novel treatments into patient care pathways. Public awareness campaigns and specialized treatment centers further drive growth in the German market.
Asia-Pacific GM1 Gangliosidosis Market Insight
The Asia-Pacific GM1 gangliosidosis market is poised to grow at the fastest CAGR of 23.7% during the forecast period, driven by increasing healthcare awareness, improving access to rare disease treatments, and rising investments in genetic disorder research across countries like India, China, and Japan. Government initiatives supporting rare disease management, coupled with expanding healthcare infrastructure and patient assistance programs, are fueling demand for advanced therapies. Growth is further supported by collaborations between local and global pharmaceutical companies and the expansion of diagnostic and treatment facilities in major urban centers.
Japan GM1 Gangliosidosis Market Insight
The Japan GM1 gangliosidosis market is gaining momentum due to the country’s strong focus on advanced healthcare, high investment in rare disease research, and widespread adoption of innovative therapeutic protocols. Specialized treatment centers, patient registries, and active government support programs are improving disease management and patient outcomes. In addition, collaborations with global biotech firms and ongoing clinical trials are contributing to the expansion of available therapies and market growth.
China GM1 Gangliosidosis Market Insight
The China GM1 gangliosidosis market accounted for the largest revenue share within the Asia-Pacific region in 2024, attributed to rapid healthcare modernization, government-led initiatives to improve rare disease management, and increased investment in hospital infrastructure. China’s expanding healthcare access, growing awareness of genetic disorders, and emphasis on early diagnosis and advanced therapies are driving demand. Partnerships with multinational pharmaceutical companies, expanding clinical trial activities, and rising adoption of gene therapy and other targeted treatments further support market growth in China.
GM1 Gangliosidosis Market Share
The GM1 Gangliosidosis industry is primarily led by well-established companies, including:
- Oracle (U.S.)
- Sanofi (France)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Orchard Therapeutics (U.K.)
- Amicus Therapeutics (U.S.)
- Shire Pharmaceuticals (Ireland)
- Biomarin Pharmaceutical Inc. (U.S.)
- Moderna Therapeutics (U.S.)
- Sangamo Therapeutics (U.S.)
- Roche Holding AG (Switzerland)
- Bluebird Bio, Inc. (U.S.)
- Precision Biosciences (U.S.)
- Avrobio, Inc. (U.S.)
Latest Developments in Global GM1 Gangliosidosis Market
- In August 2023, Passage Bio announced promising interim clinical data from the first eight patients treated with PBGM01 in the Imagine-1 study. The data showed that Dose 2 resulted in substantial improvements in key cerebrospinal fluid (CSF) biomarkers, achieving normal levels of β-galactosidase activity and GM1 gangliosides, similar to healthy controls. These improvements demonstrated durability up to 12 months after treatment. In addition, treated patients showed initial evidence of improved survival compared to natural history data
- In January 2025, Azafaros B.V. announced that its lead asset, nizubaglustat, received Orphan Drug Designation from the U.S. FDA and Orphan Medicinal Product Designation from the European Medicines Agency for the treatment of GM1 gangliosidosis. The company's Clinical Trial Application (CTA) for two global Phase 3 studies investigating the drug's efficacy and safety in GM1/GM2 gangliosidoses and Niemann-Pick Type C (NPC) was approved by multiple European countries. The Phase 3 studies were scheduled to initiate in 2025
- In July 2025, a Phase 1-2 study published in medRxiv provided evidence that a single intravenous infusion of AAV9 gene therapy carrying β-galactosidase was well-tolerated among the first nine Type II GM1 gangliosidosis participants. Secondary and exploratory outcomes suggested improvements in biochemical markers and neuroimaging, with stabilized or reduced rates of developmental deterioration
- In April 2025, a study published in The Journal of Clinical Investigation highlighted the combination of an adeno-associated virus (AAV) vector expressing a blood-brain barrier–penetrating peptide as a promising approach for GM1 gangliosidosis treatment. This combination showed significant advantages, including the potential for continuous enzyme secretion without the need for repeated administrations, addressing the challenge of delivering therapies across the blood-brain barrier
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.